5024977
C6 - Chemical Synthesis
Beschreibung
Mindmap von Alice Hathaway, aktualisiert more than 1 year ago
Mehr
Weniger
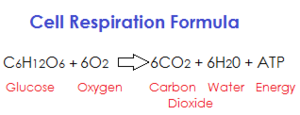
|
Erstellt von Alice Hathaway
vor mehr als 8 Jahre
|
|
Zusammenfassung der Ressource
C6 - Chemical Synthesis
- Chemical Industry
- Food additives
- Cleaning products
- Bleach/ oven cleaner/ washing up
liquid developed by chemists
- Bleach/ oven cleaner/ washing up
liquid developed by chemists
- Drugs
- Pharmaceutical industry is huge
- Pharmaceutical industry is huge
- Fertilisers
- Contain lots of
ammonia, produced
by chemical industry
- Contain lots of
ammonia, produced
by chemical industry
- Decorating products
- Paints etc contain
pigments and dyes
- Paints etc contain
pigments and dyes
- Large Scale
- Need lots of
- Ammonia
- Sulfuric acid
- Sodium hydroxide
- Phosphoric acid
- Ammonia
- Need lots of
- Small Scale
- Still important but
less is needed
- Pharmaceuticals
- Has largest share of industry
- Has largest share of industry
- Fragrance
- Flavourings
- Pharmaceuticals
- Still important but
less is needed
- Some sold directly to
consumers, some sold to
industries as raw products
- Food additives
- Acids and Alkalis
- Pure acidic compounds
- Solid e.g. citric acid
- Liquid e.g. sulfuric acid
- Gas e.g. hydrogen chloride
- Solid e.g. citric acid
- Common alkalis
- Sodium hydroxide e.g. bleach
- Potassium hydroxide
e.g. alkaline batteries
- Calcium hydroxide e.g. neutralise acidic soils
- Sodium hydroxide e.g. bleach
- Pure acidic compounds
- Indicators/ pH meters tell us pH
- Litmus paper
- Alkali
- Acid
- Alkali
- Universal Indicator
- Combination of dyes allow pH estimation
- Combination of dyes allow pH estimation
- pH Meter
- Probe and meter
- Accurate pH reading
- More accurate than indicators
- Accurate pH reading
- Probe and meter
- Litmus paper
- Acid
- pH< 7
- H+ ions in water
- Aqueous hydrogen ions
- pH< 7
- Alkali
- pH> 7
- Aqueous hydroxide ions
- OH - ions in water
- pH> 7
- Neutralisation
- H+(aq) + OH- (aq) ---> H2O (aq)
- Acid + Alkali ---> Salt + Water
- pH 7 - not acid or alkali (neutral)
- H+(aq) + OH- (aq) ---> H2O (aq)
- Acids with Metals
- Acid + Metal --> Salt + Hydrogen
- More reactive = faster reaction
- Copper doesn't react as it's less reactive
than hydrogen
- Speed
determined by
rate of
hydrogen
bubbles given
off
- Copper doesn't react as it's less reactive
than hydrogen
- Hydrogen is confirmed through lit splint
- Squeaky pop
- Squeaky pop
- More reactive = faster reaction
- Acid + Metal Carbonate--> Salt + Water + Carbon Dioxide
- Hydrochloric acid + sodium carbonate --> sodium chloride + water + carbon dioxide
- Sulfuric acid + calcium carbonate --> calcium sulfate + water + carbon dioxide
- Hydrochloric acid + sodium carbonate --> sodium chloride + water + carbon dioxide
- Acid + Metal Hydroxide --> Salt + Water
- Hydrochloric acid + sodium hydroxide --> sodium hydroxide + water
- Sulfuric acid + calcium hydroxide --> calcium sulfate + water
- Hydrochloric acid + sodium hydroxide --> sodium hydroxide + water
- Acid + Metal Oxide --> Salt + Water
- Hydrochloric adid + magnesium oxide --> magnesium chloride + water
- Sulfuric acid + Zinc oxide --> zinc sulfate + water
- Hydrochloric adid + magnesium oxide --> magnesium chloride + water
- Sulfuric Acid produces Chloride Salts
- Sulphuric acid + magnesium --> magnesium sulfate + hydrogen
- Sulfuric acid + aluminium --> aluminium sulfate + hydrogen
- Sulphuric acid + magnesium --> magnesium sulfate + hydrogen
- Hydrochloric Acid produces Sulphate Salts
- Hydrochloric acid + magnesium --> magnesium chloride + hydrogen
- Hydrochloric acid + aluminium --> aluminium chloride + hydrogen
- Hydrochloric acid + magnesium --> magnesium chloride + hydrogen
- The combination of metal and acid
decides the salt
- Acid + Metal --> Salt + Hydrogen
- Synthesising Compounds
- 7 stages
- 1) Choosing the Reaction
- 2) Risk Assessment
- 3) Calculating Quantities of Reactants
- 4) Choosing the Apparatus and Conditions
- 5) Isolating the Product
- 6) Purification
- 7) Measuring Yield and Purity
- Tells overall success of reaction
- Compares total possible amount with amount actually received
- Actual yield = Mass of pure product received
- Theoretical yield = maximum possible mass could get
- PERCENTAGE YIELD = ACTUAL YIELD/ THEORETICAL YIELD X 100
- Actual yield = Mass of pure product received
- Purity must be measured
- How much actual substance
- How much actual substance
- High yield and purity wanted
- Tells overall success of reaction
- Isolating helps purification
- Crystalilsation
- 7) Measuring Yield and Purity
- Finished products separate from reaction mixture
- Evaporation if product is dissolved
- Filtration if insoluble solid
- Separate solid from liquid
- Separate solid from liquid
- Drying to remove water
- Drying oven
- Hot, dry air
- Hot, dry air
- Desiccaotr
- Chemicals e.g. silica gel to remove water
- Chemicals e.g. silica gel to remove water
- Drying oven
- 6) Purification
- Correct size
- Hold all reactants and products
- Hold all reactants and products
- Correct strenth
- For type of reaction e.g. hot temperatures
- For type of reaction e.g. hot temperatures
- Temperature of reaction
- Concentration of reactants
- If catalyst needed
- 5) Isolating the Product
- How much needed to produce required amount of product
- How much raw material is needed
- Reduce waste/ money loss
- 4) Choosing the Apparatus and Conditions
- Identifying hazards
- Who may be harmed
- What action can reduce risk
- 3) Calculating Quantities of Reactants
- Which reaction you're using
- Neutralisation - acid and alkali
react to produce a sale
- Thermal decomposition - heat used
to break a compound into simpler
substances
- Precipitation - an insoluble solid is formed
when 2 solutions are mixed
- 2) Risk Assessment
- 7 stages
- Mass Calculations
- Work out masses
needed/ theoretical
amounts of reactions
- Purity
- Isolation improves the purity of the product
- Repeating filtration/ evaporation/ crystallisation will improve this
- Repeating filtration/ evaporation/ crystallisation will improve this
- The purity required depends on the product
- High purity
- Petrochemicals
- Impurities can cause
damage to engines
- Impurities can cause
damage to engines
- Pharmaceuticals
- Drugs for human
consumption must be pure so
they don't harm
- Drugs for human
consumption must be pure so
they don't harm
- Petrochemicals
- Little purity required
- Fertilisers
- Fertilisers
- High purity
- Isolation improves the purity of the product
- Energy Transfer in Reactions
- Exothermic
- More bonds made than broken
- Energy given out
- Increase temperature
- Increase temperature
- Energy given out
- Products lower energy than reactants
- Controlling reactions
- Heat must be removed
- Temperature will increase
- Temperature will increase
- Rate of reaction will also increase and
reaction will get hotter
- Too hot = increase rate/
uncontrollable/ explosion
- Heat must be removed
- More bonds made than broken
- Endothermic
- More bonds broken than made
- Energy taken in
- Temperature decrease
- Temperature decrease
- Energy taken in
- Products higher energy than reactants
- Controlling reaction
- Heat needs to be provided to continue reaction
- Slow down rate
- Mix could freeze, damaging
equipment and stopping process
- Heat needs to be provided to continue reaction
- More bonds broken than made
- Exothermic
- Rates of Reaction
- How quickly reactants turned into products
- Slow
- Rusting iron
- Chemical weathering
(acid rain)
- Rusting iron
- Fast
- Burning
- Explosion
- Burning
- Moderate
- Metal with acid
- Metal with acid
- Slow
- Controlling the Rate
- Safety
- Too quick = explosion
- Dangerous
- Dangerous
- Too quick = explosion
- Economic
- High rate = increase temperature
- Costs to maintain
- More product in less time
- Costs to maintain
- Choose optimum
conditions for low
costs
- Compromise on rate
of production/yield
- Compromise on rate
of production/yield
- Want best yield and rate of production for lowest cost
- High rate = increase temperature
- Safety
- Increasing rate:
- Larger surface area
- More exposed particle to react
- More exposed particle to react
- Increase concentration
- More particles to react
- More particles to react
- Increase temperature
- More energy = faster moving =
more frequent collision
- More energy = faster moving =
more frequent collision
- Add catalyst
- Surface to stick to to increase collision chance
- Surface to stick to to increase collision chance
- More collisions = higher reaction rate
- Larger surface area
- How quickly reactants turned into products
- Measuring rates of Reaction
Medienanhänge
- f47273ea-48c9-47ba-a4a9-be3c8959b3ec.JPG (image/JPG)
- 71a8167a-fff4-4aac-b80c-c58a196447cb (image/png)
- 5d8846d2-0f00-445c-9411-ee6d391bead0 (image/png)
- 459a26a4-252b-4130-9fee-77f1f9c5a932 (image/jpg)
- 6af9c917-de9c-4973-b1ee-1b49f8a03511 (image/jpg)
- d7cba450-6eaa-459a-96d8-eca872c42d61 (image/png)
- e373f863-5859-4fa3-88e4-5130cf50d449 (image/png)
- 5f86dc60-02eb-491c-bee3-16f1f84a9b0b (image/png)
- dede7291-35ae-4c2e-9f60-bd6af5a42ee7 (image/jpg)
Möchten Sie kostenlos Ihre eigenen Mindmaps mit GoConqr erstellen? Mehr erfahren.
