Karteikarten am reaction rate, erstellt von Alvaro Vargas Calero am 02/05/2018.
Angeheftet an
43
0
0
Keine Merkmale angegeben
|
|
Erstellt von Alvaro Vargas Calero
vor mehr als 6 Jahre
|
|
Schließen
|
|
Erstellt von Alvaro Vargas Calero
vor mehr als 6 Jahre
|
|

Reaction rate

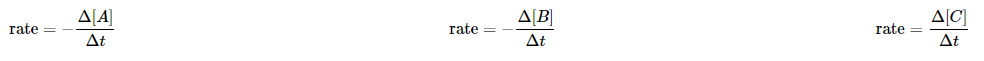
in which Δ[A] is the difference between the concentration of A over the time interval t2 – t1:
Notice the minus signs in the first two examples above. The concentration of a reactant always decreases with time, so Δ[A] and Δ[A] are both negative.
Consider now a reaction in which the coefficients are different:
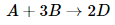
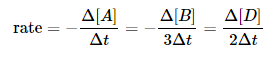

 Verberge bekannte Karten
Verberge bekannte Karten