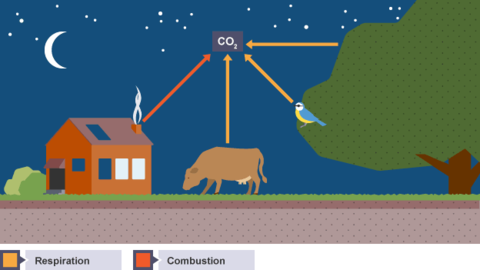
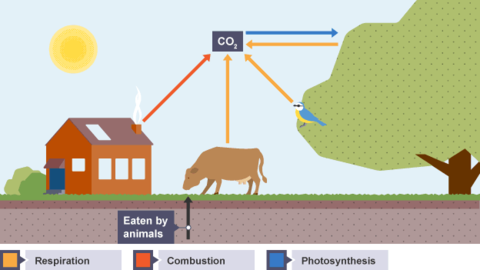
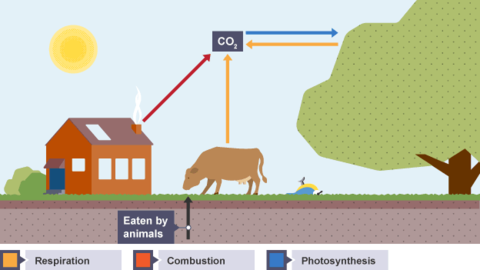
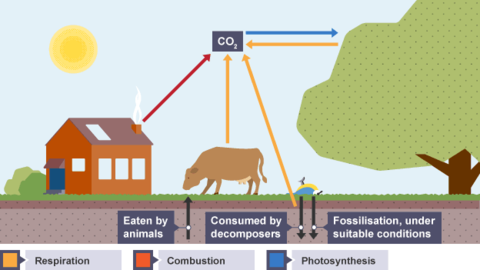
Karteikarten am Products and effects of combustion, erstellt von LividOatmeal am 09/03/2015.
Angeheftet an
30
1
0
Keine Merkmale angegeben
|
|
Erstellt von LividOatmeal
vor mehr als 9 Jahre
|
|
Schließen