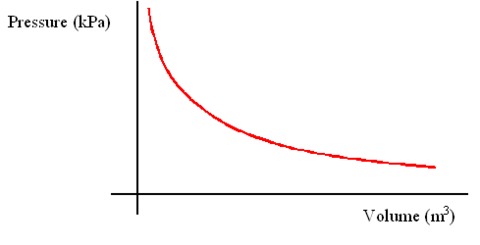
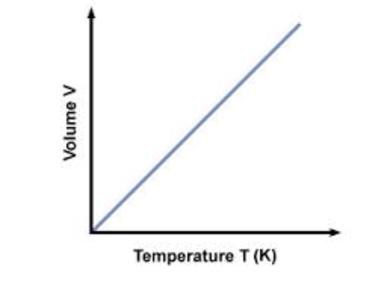
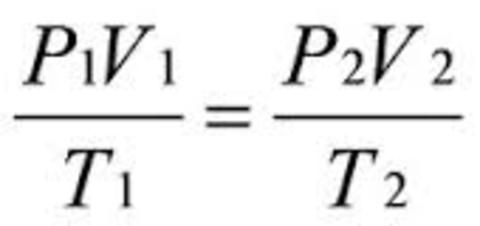Leaving Certificate Chemistry (Gas Laws And The Mole) Karteikarten am Gas Laws And The Mole, erstellt von eimearkelly3 am 22/09/2013.
Angeheftet an
479
6
0
Keine Merkmale angegeben
|
|
Erstellt von eimearkelly3
vor etwa 11 Jahre
|
|
Schließen