- the construction & operation of electrolytic cells
Angeheftet an
10
0
0
Keine Merkmale angegeben
|
|
Erstellt von Fiona Heathcote
vor etwa 9 Jahre
|
|
Schließen
|
|
Erstellt von Fiona Heathcote
vor etwa 9 Jahre
|
|

Electrolysis involves the passage of electricity through a conducting liquid to cause a _______ reaction to occur
During electrolysis ___________ energy is converted into ____________ energy
Electrolysis involves spontaneous/non-spontaneous reactions
Electroplating is ...
Electroplating is performed in an _____________ cell
The object to be plated (to have a thin layer of metal deposited on it) is connected to the positive/negative terminal of the power supply
The object to be plated is regarded as the positive/negative electrode
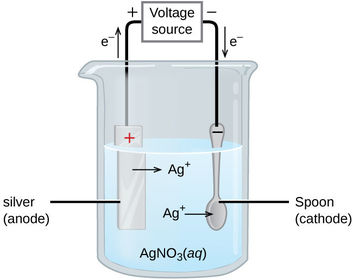
What is the half equation for the reaction at the anode?
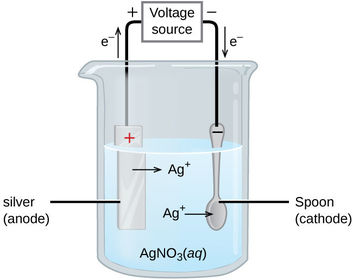
What is the half equation for the reaction at the cathode?
In an electrolytic cell, both the anode and the cathode are immersed in a solution known as an ______________
An electrolyte is...
When an electrolytic cell is working, the power supply acts as an "electron pump".
Electrons are pushed onto the ___________ electrode and are removed from the _____________ electrode.
What reaction occurs at the negative electrode during electrolysis?
What reaction occurs at the positive electrode during electrolysis?

 Verberge bekannte Karten
Verberge bekannte Karten