using entropy and enthaply to discuss the spontaneity of a reaction
Angeheftet an
39
3
0
Keine Merkmale angegeben
|
|
Erstellt von Tanya Haywood
vor etwa 9 Jahre
|
|
Schließen
|
|
Erstellt von Tanya Haywood
vor etwa 9 Jahre
|
|

Whether a reaction is spontaneous
is dependent on the changes in
which two concepts?
Entropy is a measure of
Increasing entropy comes from
Increasing the temperature of a system
does what to the entropy?
if enthalpy change is positive
compare the relative enthalpy of the
reactants with the products
if enthaply change is positive
describe what you would feel
as the reaction proceeds
Sponaneity is favoured with
what change in enthaply?
Describe an exothermic reaction
spontaneous or not?
Entropy increases
and
enthaply change is negative
spontaneous or not?
Entropy decreases
and
enthaply change is negative
spontaneous or not?
Entropy increases
and
enthaply change is positive
spontaneous or not?
Entropy decreases
and
enthaply change is positive
exo or endo??
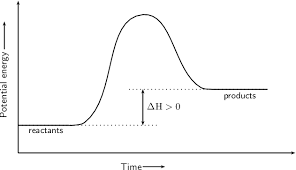
why is this endothermic?
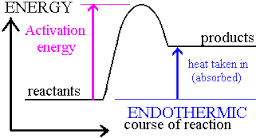
which state changes are exothermic?


 Verberge bekannte Karten
Verberge bekannte Karten