Schließen

What is the definition of a
compound?
Why do atoms take part in
chemical bonding?
When an atom loses an
electron, what charge does the
remaining ion have?
When an atom gains an
electron, what charge does the
remaining ion have?
What can we say about the
electronic structure of ions?
What do we call the elements
in Group 1
When Group 1 metals react
with non-metal elements, what
type of ion is formed?
What do we call the elements
in Group 7?
When Group 7 elements react
with alkali metals, what charge
does the halide ion gain?
What type of structure is
formed by ionic compounds?
What type of bonding happens
when atoms share pairs of
electrons?
What do we call covalent
molecules with only a small
number of atoms e.g. H2, Cl2,
O2, HCl, H20, NH3 and CH4?
What can we say about the
melting and boiling points of
simple molecules?
Why do simple molecules
have low melting and boiling
points?
Why do simple molecules not
conduct electricity?
Why do ionic compounds have
high melting and boiling
points?
Why do solid ionic compounds
not conduct electricity?
Why do ionic compounds
conduct electricity when they
are melted or dissolved in
water?
What type of molecules are
diamond, graphite and silicon
dioxide?
Why do giant covalent
structures have very high
melting points?
Damond and graphite are
formed from which element?
What is this molecule?
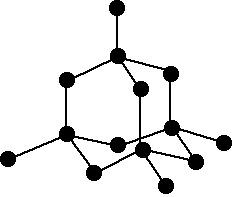
What is this molecule?
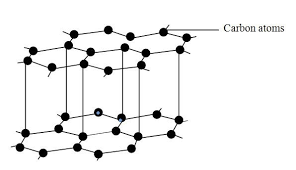
Why is diamond very hard?
Why is graphite soft and
slippery?
Why is graphite a good
conductor of heat and
electricity? (HIGHER TIER)
What is the structure of
fullerenes? (HIGHER TIER)
Why are metals good
conductors of heat and
electricity? (HIGHER TIER)
Why can metals be bent and
shaped?
What are alloys?
Why are alloys harder than
pure metals?
What are shape memory
alloys?
What conditions can we
change to alter the properties
of a polymer?
What is meant by a
thermosoftening polymer?
What is meant by a
thermosetting polymer?
In terms of intermolecular
forces, why can
thermosoftening polymers melt
when heated? (HIGHER TIER)
What is meant by
nanoparticles?
Why are nanoparticles useful?
(a role may be described in the exam).
What is shown by the atomic
number of an element?
What is shown by the mass
number of an element?
How do we calculate the
number of neutrons in the
nucleus of an atom?
What do we call atoms of the
same element with different
numbers of neutrons?
What is meant by the relative
atomic mass (Ar) of an
element? (HIGHER TIER)
What is meant by the relative
formula mass (Mr) of a
compound?
What is one mole of a
substance?
Elements and compounds can
be detected and identified by
instrumental methods. What
does this mean and give an
example.
What are the advantages of
analysing a substance using
an instrumental method?
How can we identify artificial
colours in foods?
Describe how gas
chromatography works.
How can we tell from a gas
chromatograph how many
different compounds are
present?
How can we work out the
retention time from a gas
chromatograph?
How can we work out the
relative molecular mass of a
substance from a mass spectrum?
(HIGHER TIER)
Suggest why the yield of a
reaction is often less than the
maximum calculated amount.
How do we calculate the
percentage yield for a reaction.
What does this symbol mean?
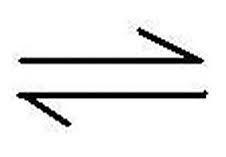
How can we calculate the rate
of a reaction?
What is meant by the
activation energy?
How does increasing the
temperature of a reaction
increase the rate of that
reaction?
How does increasing the
pressure of reacting gases
increase the rate of a
reaction?
How does increasing the
concentration of reactants in
solution increase the rate of
reaction?
How does increasing the
surface area of solid reactants
increase the rate of reaction?
What is the effect of a catalyst
on a chemical reaction?
How can using a catalyst save
money when carrying out a
chemical reaction?
What is meant by an
exothermic reaction?
What is meant by an
endothermic reaction?
How can we use anhydrous
copper sulfate to test for the
presence of water?
What are the state symbols for
• solid
• liquid
• gas
• dissolved in water
Describe how we can make a
soluble salt by reacting a metal
with an acid.
Describe how we can make a
soluble salt by reacting an
insoluble base with an acid.
Describe how we can make a
soluble salt by reacting an
alkali with an acid.
When we mix two solutions
together and produce a solid,
what name do we give to the
solid?
What is an alkali?
Suggest a pH value for an
acid.
What pH is a neutral solution?
Which ion makes solutions
acidic?
Which ion makes solutions
alkaline?
What is the equation for
neutralisation?
Which salts are produced
from:
• hydrochloric acid
• sulfuric acid
• nitric acid
How is ammonium nitrate
produced and what is it used
for?
What is meant by electrolysis?
In electrolysis, what is meant
by the electrolyte.
During electrolysis, which ion
moves to the negative
electrode and why?
Suggest a reason why we
electroplate a metal with a
different metal.
During electrolysis, what takes
place at the negative
electrode?
During electrolysis, what takes
place at the positive
electrode?
When electrolysing an ionic
compound in solution, how do
we work out what is produced
at the negative electrode?
Write the half equation for the
oxidation of chloride at the
positive electrode. (HIGHER
TIER)
What is the role of cryolite
during electrolysis of molten
aluminium oxide?
During the electrolysis of molten
aluminium oxide, what is
produced at the negative
electrode and what is produced
at the positive electrode?
During electrolysis of molten
aluminium oxide, why does the
positive electrode need to be
replaced regularly?
During electrolysis of sodium
chloride solution, what is
produced at the negative
electrode?
During electrolysis of sodium
chloride solution, what is
produced at the positive
electrode?
During electrolysis of sodium
chloride, what solution remains
at the end?

 Verberge bekannte Karten
Verberge bekannte Karten