A level Chemistry (3.2 Physical Chemistry) Flashcards on Enthalpy Changes, created by Yinka F on 04/03/2018.
Pinned to
444
0
0
No tags specified

|
Created by Yinka F
over 6 years ago
|
|
Close

|
Created by Yinka F
over 6 years ago
|
|

What is enthalpy change?
What does this symbol mean:
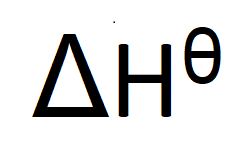
What are the standard conditions?
You can't directly measure the actual enthalpy of a system. What is used instead?
Complete the sentences:
Enthalpy changes in data books are usually _____________ enthalpy changes. This is important as changes in enthalpy are affected by ______________ and ____________.
What does this symbol mean:
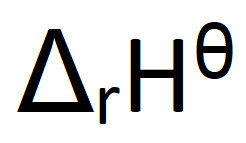
Define standard enthalpy change of reaction
What does this symbol mean:
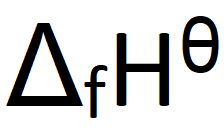
Define standard enthalpy change of formation
What does this symbol mean:
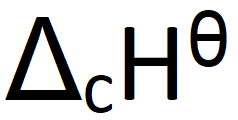
Define standard enthalpy change of combustion
What does this symbol mean:
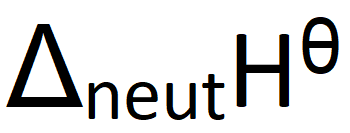
Define standard enthalpy change of neutralisation
Complete the sentences:
Exothermic reactions _______ ______ energy to their surroundings. The products of the reaction end up with _______ energy than the reactants. This means that the enthalpy change for the reaction will be ___________.
TRUE OR FALSE:
Oxidation is usually exothermic
Describe what an enthalpy diagram of an exothermic reaction looks like
Complete the sentences:
Endothermic reactions ______ ____ energy from their surroundings. This means that the products of the reaction have ______ energy than the reactants, so the enthalpy change for the reaction is ___________.
TRUE OR FALSE:
Thermal decomposition is endothermic
Describe what an enthalpy diagram of an endothermic reaction looks like

 Hide known cards
Hide known cards