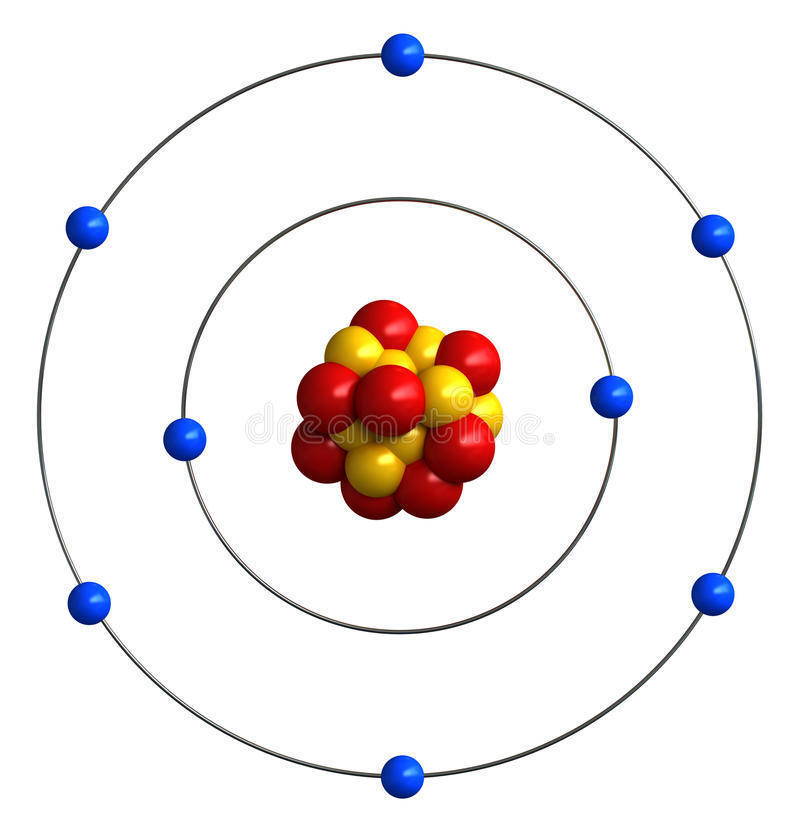
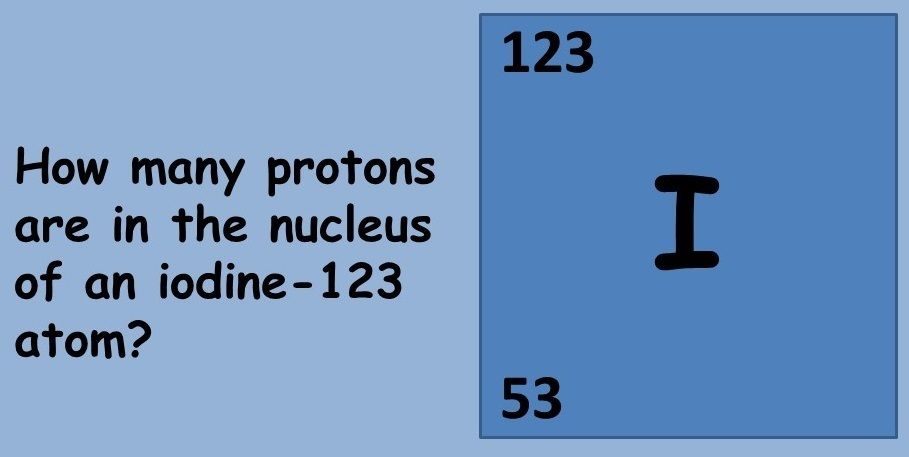
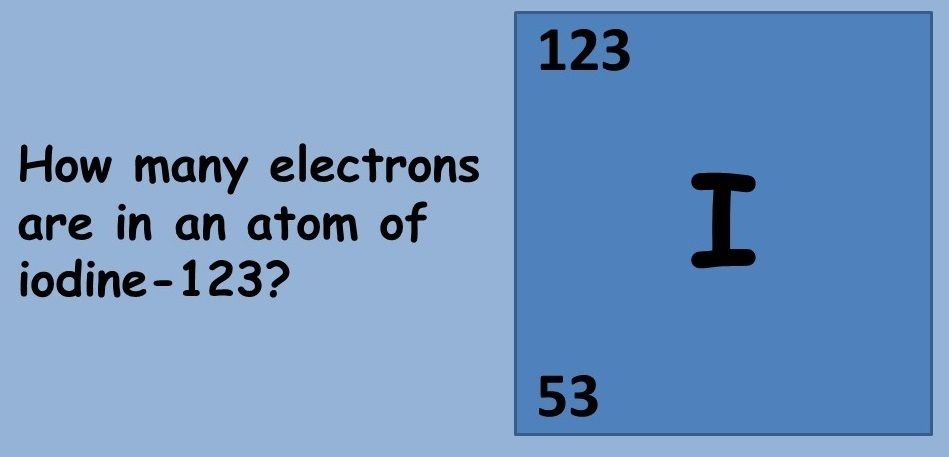
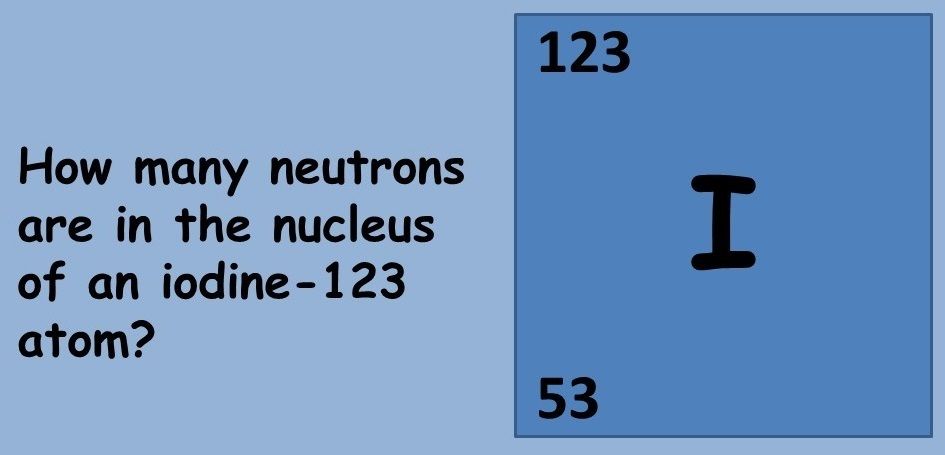
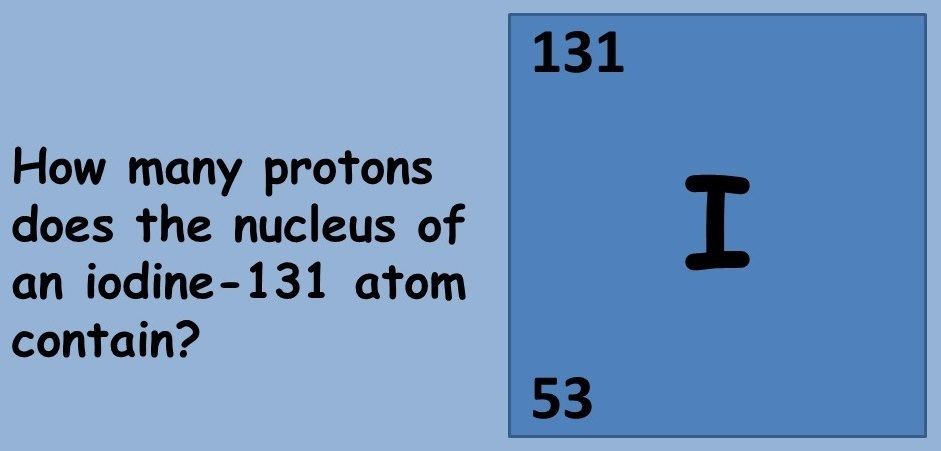
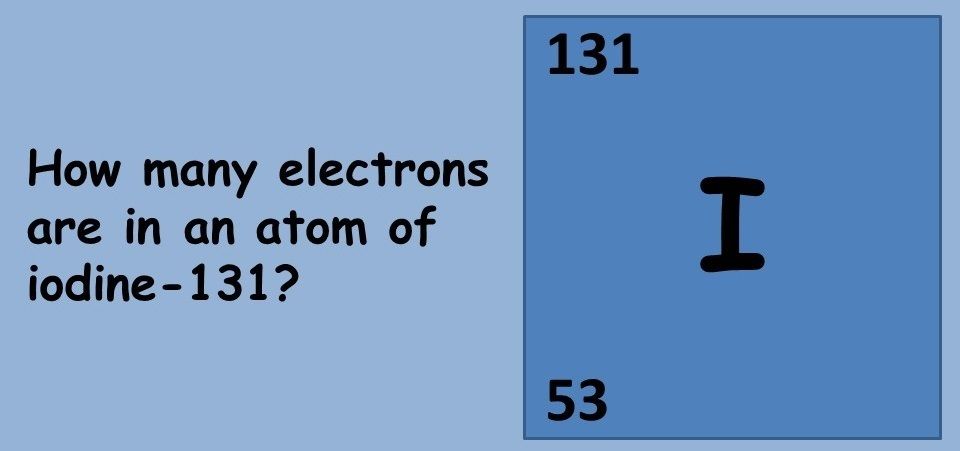
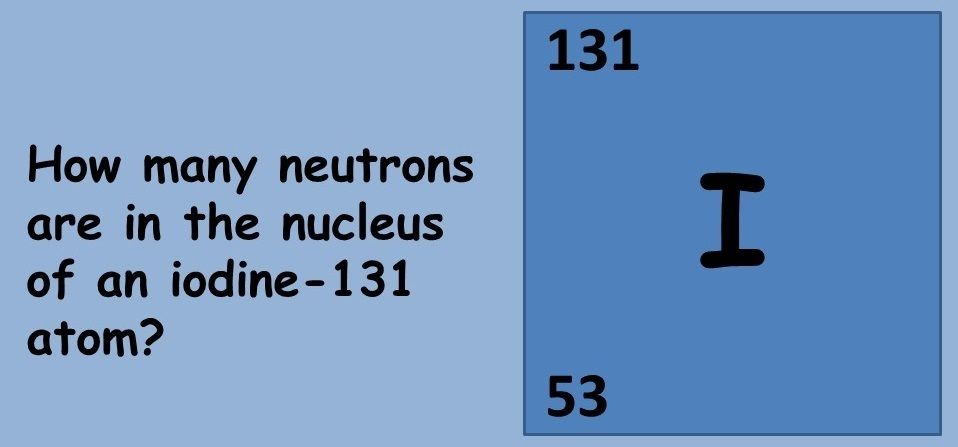
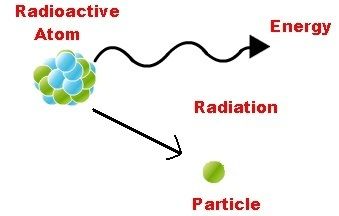
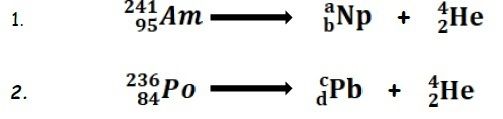
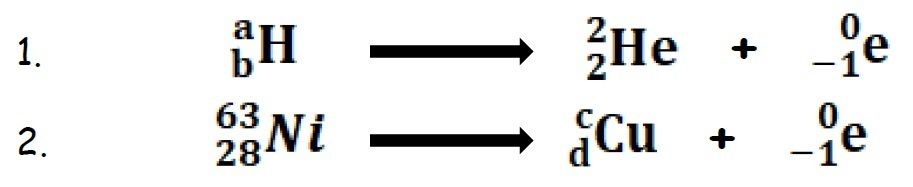
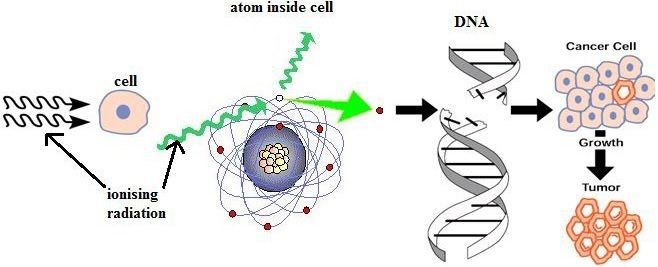
This topic covers the structure of the nuclear atom and its representation using atomic notation. It covers the spontaneous nature of nuclear decay and the nature of alpha, beta and gamma radiation. Learners will produce and balance nuclear equations for radioactive decay.
Pinned to
311
0
0
No tags specified
|
Created by Mr S Lee
over 6 years ago
|
|
Close