Flashcards on Activation Energy, created by Alvaro Vargas Calero on 30/04/2018.
Pinned to
56
0
0
No tags specified
|
|
Created by Alvaro Vargas Calero
over 6 years ago
|
|
Close
|
|
Created by Alvaro Vargas Calero
over 6 years ago
|
|

Activation energy
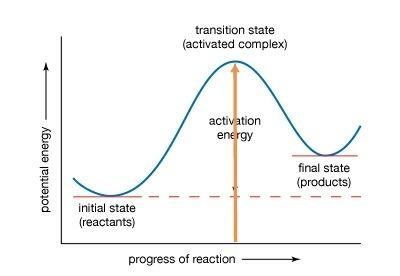
Activation energy can be thought of as the height of the potential barrier (sometimes called the energy barrier) separating two minima of potential energy (of the reactants and products of a reaction). For a chemical reaction to proceed at a reasonable rate, there should exist an appreciable number of molecules with translational energy equal to or greater than the activation energy.
The term was introduced in 1889 by the Swedish scientist Svante Arrhenius.

 Hide known cards
Hide known cards