Flashcards on nonspontaneous reaction, created by Alvaro Vargas Calero on 02/05/2018.
Pinned to
57
0
0
No tags specified
|
|
Created by Alvaro Vargas Calero
over 6 years ago
|
|
Close
|
|
Created by Alvaro Vargas Calero
over 6 years ago
|
|

Nonspontaneous reaction
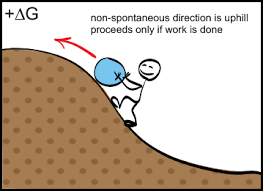
Out atmosphere is composed primarily of a mixture of nitrogen and oxygen gases. One could write an equation showing these gases undergoing a chemical reaction to form nitrogen monoxide.
Fortunately, this reaction is nonspontaneous at normal temperatures and pressures. It is a highly endothermic reaction with a slightly positive entropy change (ΔS) . However, nitrogen monoxide is capable of being produced at very high temperatures, and this reaction has been observed to occur as a result of lightning strikes.

 Hide known cards
Hide known cards