Flashcards on Physics GCSE Triple Science OCR P6, created by saffiehermione on 02/10/2014.
Pinned to
125
4
0
No tags specified
|
|
Created by saffiehermione
about 10 years ago
|
|
Close
|
|
Created by saffiehermione
about 10 years ago
|
|

P6: RADIOACTIVE MATERIALS
All elements are made of one type of atom. All atoms contain a nucleus and electrons.
The nucleus is made from protons and neutrons. With the exception of Hydrogen (lightest element) which has just one proton and electron.
Radioactive materials can emit three types of ionising radiation:
Alpha
Beta
Gamma
Radioactive Decay: Ionising radiation is emitted when the nucleus of an unstable atom decays. The type of radioactive decay depends on why the nucleus is unstable. The process of decay helps helps the atom become stable. During this the proton number may change causing the element to change type.
(B) The atom converts a neutron into a proton and an electron. The electron is ejected. Proton stays in nucleus changing element.
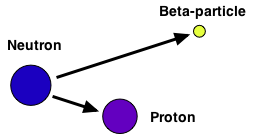
Background Radiation: Radioactive elements are found naturally in the environment and contribute to background radiation. The levels are usually so low there is no danger. However there are correlations with certain cancers and living in specific areas.

 Hide known cards
Hide known cards