- Property, state, process and cycle - Forms of energy
Pinned to
8
0
0
No tags specified
|
|
Created by Alice Kimpton
almost 6 years ago
|
|
Close
|
|
Created by Alice Kimpton
almost 6 years ago
|
|

What is a property?
What are intensive or extensive properties units?
What is an intensive property?
What is a specific property?
What are extensive property?
What is 'state'?
What is equilibrium state?
How are the number of properties required to fix the state of a system given?
Define the above.
Why is a system called a simple compressible system?
Define cycle.
Define process.
What is the equilibrium process?
What is the quasi-equilibrium process?
How is a non-quasi equilibrium process denoted?
What is steady flow process?
What is a thermodynamic unit?
What are macroscopic forms of energy?
Define kinetic energy.
What is the equation?
Define potential energy.
What is the equation?
What are microscopic forms of energy?
What is internal energy?
Internal energy:
-Sensible energy (HEAT / THERMAL ENERGY)
Internal energy:
-Latent energy (HEAT / THERMAL ENERGY)
Internal energy:
- Chemical energy
- Nuclear energy
Total energy definition and equation.
How does thermodynamics use total energy?
What can total energy be assigned?
What is the change in energy independent of?
What is energy transfer?
Energy transfer: heat?
Energy transfer: work?
Similarities between heat and work energy transfers?
What is mass flow?
When direction of heat or work is not known, what do we do?
What do +ve and -ve results indicate?
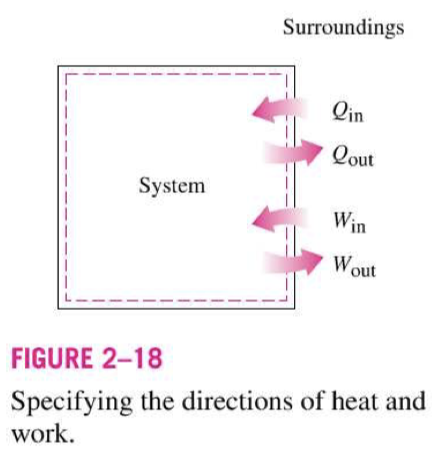
State the first law of thermodynamics / energy principle.
What is the net change in total energy of system during a process equal to?
What is the change in total energy of a system equation?
Continued change in total energy of a system equation
What is the change in total energy of a system equation in the rate form?
What is the equation for efficiency?
Efficiency of gas boiler

 Hide known cards
Hide known cards