Close

Describe a covalent bond, example, and its electronegativity values
Describe a polar covalent bond, example, and its electronegativity values
Describe an ionic bond, example, and its electronegativity values
What is electronegativity, and how does it appear on a periodic table? What happens to the atomic radius, valence electrons and their power as the electronegativity increases (across)
Difference between anions and cations
Lewis structure method:
Formal charge formula
Octet rule
What does the 'n' quantum symbol mean?
What does the 'q' quantum symbol mean?
What does the 'ml' quantum symbol mean?
What does the 'ms' quantum symbol mean?
Difference between diamagnetic and paramagnetic
What is the Pauli exclusion principle?
What is the Aufbau principle?
What is Hund's rule?
What is VSEPR theory and what are the degrees of repulsion?
Geometry of (no lone pairs)
sp hybridised
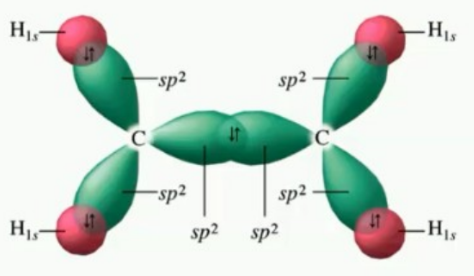
sp2 hybridised
sp3 hybridised
sp3d h
sp3d2
Geometry of
sp3 hybridised (1 lone pair)
sp3 hybridised (2 lp)
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 2 electron pairs, no lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 3 electron pairs, no lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 3 electron pairs, 1 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 4 electron pairs, no lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 4 electron pairs, 1 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 4 electron pairs, 2 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 5 electron pairs, no lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 5 electron pairs, 1 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 5 electron pairs, 2 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 5 electron pairs, 3 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 6 electron pairs, no lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 6 electron pairs, 1 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 6 electron pairs, 2 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 6 electron pairs, 3 lone pairs
What is the electron-pair geometry, molecular geometry and bond angle of:
A molecule with 6 electron pairs, 4 lone pairs
What are the five main electron pair geometry names from 2-6
What are axial and equatorial sites
Describe the overlapping and strength of sigma and pie bonds
Understand the hybridisation of this picture
Polar vs non-polar molecules
What is the shape, bond angle and types of bonds in:
sp
sp2
sp3
What are dipole moments?
What determines bond length?
What is the bond order equation?
Describe how wave functions can interact in orbitals
What is bond energy?
Rules for hybridisation
What are isomers
In uncharged molecules, state the valences for each atom:
C, O, F, Br, H, N, Cl, I
What is this group?
Suffix?
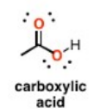
What is this group?
Suffix?

What is this group?
Suffix?
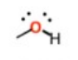
What is this group?
Suffix?
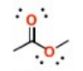
What is this group?
Suffix?
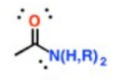
What is this group?
Suffix?
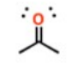
What is this group?
Suffix?
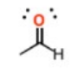
What is this group?
Suffix?
What can J*sec also be as a unit?

 Hide known cards
Hide known cards