Close

Concepts to understand
Water has several unique properties that permit life:
WATER
Polarity makes water a perfect ___ for polar substances.
Ionic solids dissolve in H2O to form ___ ions.
Polarity is also essential for the formation of micelles (soap) and bilayers (__ ___).
Non-polar substances are ___ in water.
Electrostatic interactions
1. Charge - Charge
Electrostatic interactions
2. Hydrogen Bonds
H bonds & properties of water:
-high boiling point
-high melting point
-heat of vaporization
-heat capacity
-surface tension
-viscosity
___ of H bond is important for STRENGTH
--> Strongest when ___
Partial electron pair ___ occurs b/w donor & acceptor
Hydrogen bonds ___ double helix structure in DNA
H bonds stabilize protein ___ structure (i.e. beta sheets).
Important concept: The ___ effect of many weak interactions could be a strong force
Water can form H bonds with??
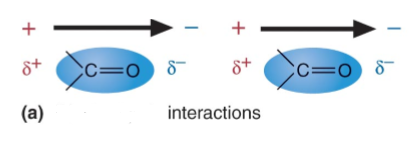
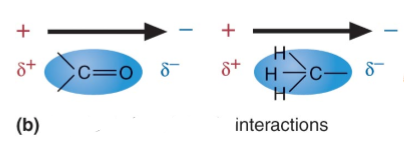
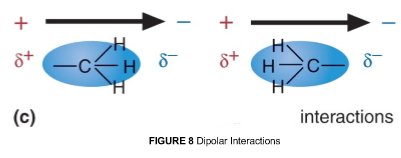
3. Van der Waals interactions
the interaction between permanent or transient __ of two molecules'.
This technically includes the hydrogen bond interactions. However, H- bond is often separated as a special case.
There are classes of interactions can be distinguished:
1. Between two dipoles (two H20 molecules)
2. Between a dipole and non-polar atoms (Debye force)
3. Between non-polar atoms (___ ___ force)
In 2 and 3, non-polar atoms get polarized by the neighbor
*Van der Waals interactions are forces that can be BOTH ___ and ___
Water auto-ionization
Because of electron-rich O atom, H20 is a ___
It attracts (rather attacks) a positively charged (electron-deficient atom).
A water molecule may attack another __ molecule to cause ionization.
--> The result is hydronium (H3O+) and hydroxide (OH-) ions.
Ion product of water
__ = ion product of water
Kw = 1X10^-14
IMPORTANT:
Kw does __ change when acid or base are added to the solution, assuming [H2O] does not change significantly.
[H+] is an important biochemical parameter
pH = -log [__]
In pure water [H+] = 10^-7 and pH = 7
In blood = about __
IN tumors pH is often <7
*The "right" pH is required for enzymes to function.
pH values depend on the degree of ____ dissolved in water molecules, such as acids and bases.
HCI is an example of a strong acid, it dissociates almost ___
Example:
If 1mM HCI is prepared, pH= -log(0.0001) =3
[H+][OH-] = 10^-14 (Kw)
**If a low concentration of acid added in a lot of water, the diluted water dominates and the pH remains close to __
(cutoff around 1 micro Molar - 10^-6)
Weak acids are only ___ dissociate.
Ka is an acid dissociation constant:
The same convenience logic as in pH, logarithmic values are used:
pKa = -log Ka
Henderson--Hasselbalch Equation
-The relation between pH and pKa
pH = pKa + log ( [proton acceptor] / proton donor])
pKa = pH when [proton acceptor] = [proton donor]
Addition of NaOH to a solution containing a weak acid and its conjugate base does not substantially change pH in the region pH=pKa ±1.
This phenomenon is called ___.
Equilibrium shifts to offset +/-[OH]
pH = pKa at the ___ of the titration curve

 Hide known cards
Hide known cards