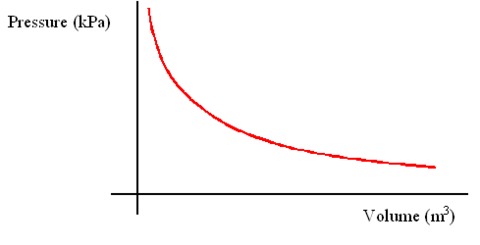
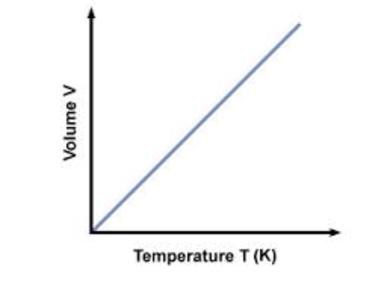
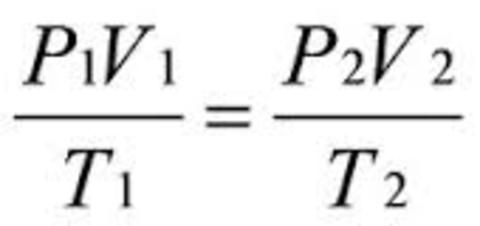Leaving Certificate Chemistry (Gas Laws And The Mole) Flashcards on Gas Laws And The Mole, created by eimearkelly3 on 22/09/2013.
Pinned to
479
6
0
No tags specified
|
|
Created by eimearkelly3
about 11 years ago
|
|
Close