Flashcards on OCR AS Chemistry A Organic Chemistry Revision, created by Miss K Hammond on 16/03/2015.
Pinned to
49
2
0
No tags specified
|
|
Created by Miss K Hammond
over 9 years ago
|
|
Close
|
|
Created by Miss K Hammond
over 9 years ago
|
|

What are hydrocarbons?
Explain what is meant by the term, 'unsaturated hydrocarbon'.
What is a functional group?
Define the term 'homologous series'.
What is the general formula for:
(i) alkanes
(ii) alkenes
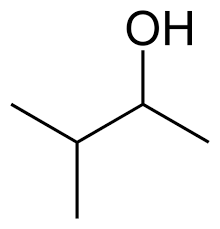
Name this organic molecule.
Give the displayed formula for butanoic acid.
Define:
(i) structural isomer
(ii) stereoisomer
Draw and label the E/Z isomers of 1,2-dichloropropene.
Define:
(i) homolytic fission
(ii) heterolytic fission
What is an electrophile?
What is a nucleophile?
What is fractional distillation?
Why do branched alkanes have lower boiling points than straight chain alkanes?
Write an equation for the incomplete combustion of octane.
What is the name of this process?
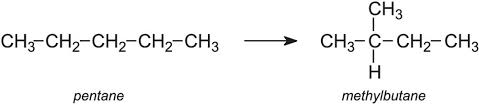
Show how pentane can be reformed.
Why are branched and cyclic alkanes used as petrol additives?
Show the (mechanism) propagation equations for the reaction of methane with chlorine.
Represent the pi bond in alkenes in a labeled diagram.
What type of reactions do alkenes always undergo?
What reagent and conditions are required for the formation of ethane from ethene?
Show the electrophilic addition mechanism for the bromination of ethene.
What reagent and conditions are required for the formation of ethanol from ethene?
Draw the two products formed in the hydration of but-1-ene and explain why there are two products.
Give an equation for the polymerization of propene.
Draw the monomer that was used to make the polymer above.
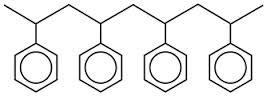
How can polymer waste be minimized?
Write an equation showing the formation of ethanol via fermentation. Give the reagents and conditions required.
Draw the hydrogen bonding between 2 ethanol molecules and use it to explain why alcohols have higher boiling points than alkanes of similar mass.
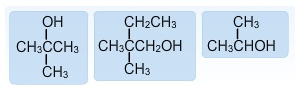
Classify the above alcohols as primary, secondary and/or tertiary.
Give equations for the 2 step reaction of ethanol with acidified potassium dichromate.
What is this reaction showing? What would you observe?
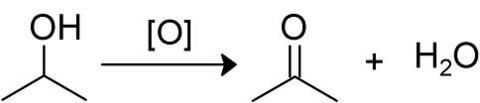
Define the term 'reflux'.
Show the esterification reaction that would form ethyl propanoate.
What conditions are required for esterification?
What are esters used for?
Show the mechanism for the nucleophilic substitution of 1-iodopropane by hydroxide ions.
Why does the hydrolysis of 1-iodobutane occur faster than for 1-chlorobutane?

 Hide known cards
Hide known cards