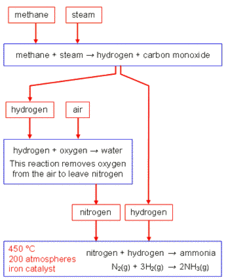
This is an overview of the whole of c2, it starts with c2.1 and goes throught to c2.7. C2.1 structure and bonding C2.2 how structure influences properties C2.3 atomic structure and quantitative chemistry C2.4 rates of reaction C2.5 exothermic and endothermic reactions C2.6 acids bases and salts C2.7electrolysis
Pinned to
607
56
0
No tags specified
|
|
Created by kristy baker
over 9 years ago
|
|
Close