Permeability of Red Blood Cell Membranes
Pinned to
270
2
0
No tags specified
|
|
Created by Afronewtzz
over 9 years ago
|
|
Close
|
|
Created by Afronewtzz
over 9 years ago
|
|

What molecules are capable of penetrating the plasma membrane? Which molecules aren't?
Give some examples of substances which can penetrate the plasma membrane, and are FULLY permeable. Why?
What is meant by lipophilic?
Why are small, POLAR molecules moderately permeant?
Name some substances which cannot cross across the membrane i.e. they are impermeant. Why? How do these molecules get into the cell?
Describe osmosis in terms of solute concentration.
If water flows throughout a water-permeable membranes from a region of high solute concentration to a region of low solute concentration until solute concentrations in both compartments are EQUAL, how does this affect the volume in each compartment?
What is a hyposomotic solution (describe in terms of osmosis)?
What is a hyperosmotic solution (describe in terms of osmosis)?
What is a isomotic solution (describe in terms of osmosis)?
Define osM (osmotic effect of a solution) in biochemistry terms.
How many moles of Glycerol does a 0.3osM solution of Glycerol contain? Why?
What is meant by a single molecular species?
How many moles of ions is in 1 mole of fully dissociated KCl?
Therefore, how many moles of KCl is in a 0.3osM solution?
Therefore, how many moles of MgCl2 is in a 0.3osM solution?
How do red blood cells appear if they are presented in a very hypotonic solution?
How do red blood cells appear if they are presented in a hypertonic solution?
How do red blood cells appear if they are presented in an isotonic solution?
How do red blood cells appear if they are presented in a very hypotonic solution?
What is meant by tonicity?
What is considered a region of low osmolarity to a RBC and why?
What is meant by osmotic haemolysis in RBCs?
How can the extent of haemolysis be measured?
What is an osmotic fragility test?
If a RBC is placed in a solution containing 0 osM impermeant solute, will its volume increase, decrease or not change? Why?
If a RBC is placed in a solution containing 1.0 osM impermeant solute, will its volume increase, decrease or not change? Why?
If a RBC is placed in a solution containing 0.3 osM impermeant solute, will its volume increase, decrease or not change? Why?
What happens if RBCs are placed in a 0.3osM solution of permeant solute?
Describe why the intracellular osmolarity of a RBC becomes greater than that of the medium when RBCs are placed into a medium of permeant solutes.
How can Hameolysis be used to determine whether a a RBC membrane is permeable to a particular compound?
If a RBC is placed in a solution containing 0.3osM impermeant solute, will its volume increase, decrease or stay the same?
If a RBC is placed in a solution containing 0.3osM permeant solute, will its volume increase, decrease or stay the same?
What is the osmolarity of a solution containing 200mM Na2PO4?
What is the osmolarity of a solution containing 200mM NaCl?
What is the osmolarity of a solution containing 0.1 M sucrose?
What is the osmolarity of a solution containing 200mM NaCl and 0.1M Na2HPO4?
Placing red blood cells in which of the following solutions is likely to lead to haemolysis?
- 0.3 M Sucrose
- 0.1 M MgCl2
- 0.1 M NaCl
- 0.2 M MgCl2
- 0.1 Na2PO4
Will adding a concentrated solution of ethanol to a red blood cell suspension cause haemolysis in the cells? What is your reasoning?
Why is ethanol permeable?
Describe the 5 stages that would follow from adding a permeant substance with high osmolarity to a red blood cell (including any tests as the end)
Describe out of the following 3 species; NaCl, Sucrose and Na2HPO4 which one requires the most moles to generate haemolysis in RBCs.
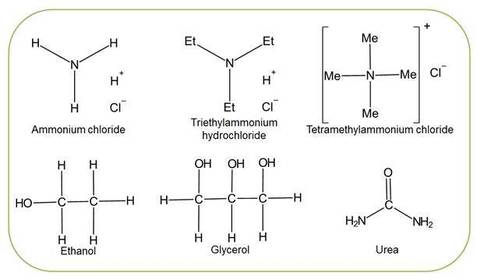
What chemical structures and properties make substances more permeable to lipid bilayer membranes?
Which of the following molecules are able to pass across the RBC membrane and enter the cell? - describe the rate at which they diffuse in if they do and their rate of haemolysis.

 Hide known cards
Hide known cards