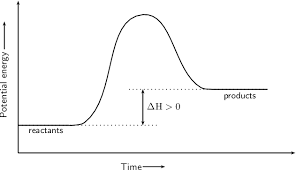
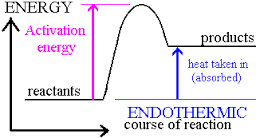
using entropy and enthaply to discuss the spontaneity of a reaction
Pinned to
1
0
0
No tags specified
|
|
Created by Tanya Haywood
almost 9 years ago
|
|
|
|
Copied by Glenn Boeckx
almost 9 years ago
|
|
Close