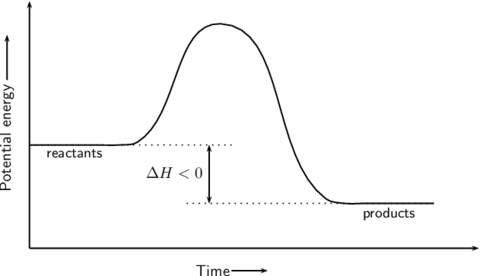
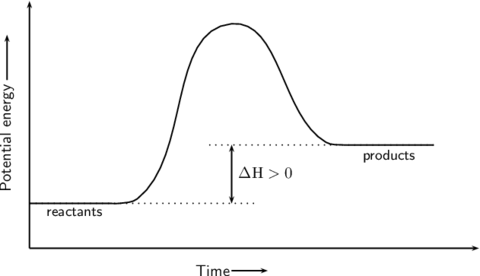
GCSE triple science Flashcards on Chemistry aqa Triple science GCSE C2, created by Vishal Kapila on 25/02/2016.
Pinned to
1
1
0
No tags specified
|
|
Created by d s
over 8 years ago
|
|
|
|
Copied by Vishal Kapila
over 8 years ago
|
|
Close