- Dry air is composed of three main gases; Nitrogen, Oxygen and Argon. It also contains trace amounts of Xenon, Neon, Hydrogen, Helium, Krypton and Carbon Dioxide.
- In general, dry air is 78.8% Nitrogen, 20.95% Oxygen, 0.93% Argon and <0.05% Xenon, Neon, Hydrogen, Helium, Krypton and Carbon Dioxide.
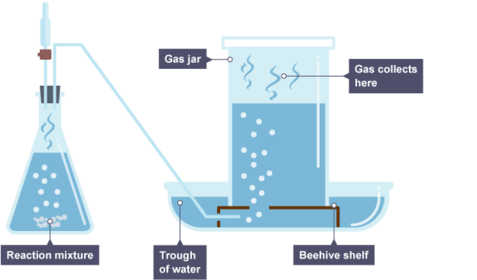
- Because Copper only reacts with Oxygen of all of the air's gases, you can prove that there is 21% Oxygen in the air by: Heating some copper, and then (between two syringes) passing air past it again and again until the volume reading of air is constant. Then you leave it to cool, subtract the lost volume of air and that volume is the volume of Oxygen. From this you can find the proportion of Oxygen in the air by dividing the lost volume over the original volume and multiplying by 100 to get a percentage.




 (16)
(16)