Close

Atoms consist of _____, _____ and _____
Protons are _____ charged
Neutrons are _____
Electrons are _____ charged
All of the elements in a _____ have the same number of _____ in their _____
Group 1 elements react with _____ to form an _____ and _____
Group 1 elements react with _____ to form _____
Group 0 elements are the _____ and all have _____ electrons in their outer shell, excluding _____
Noble gases are _____ and _____
The top number is the _____
The bottom number is the _____
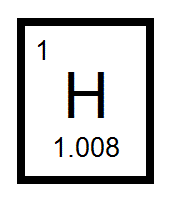
The mass number tells you the number of _____ and _____
The atomic number tells you the number of _____
Electrons always occupy _____
Atoms are more stable when then have a _____ of electrons
However, in most atoms the outer shell is not full so it wants to _____ to fill it
Compound
Making these bonds includes _____, _____ or _____ electrons
Ionic bonding
Metal atoms _____ electrons to form _____ ions
Non-metal atoms _____ electrons to form _____ ions
Describe ionic bonding to form sodium chloride
Covalent bonding
Each atom _____ electrons with the other to create a _____ of electrons
Describe covalent bonding to form hydrogen chloride
A chemical formula shows what _____ are in a _____
Limestone is mainly _____
When heated it _____ to form _____ and _____
Chemical equation for the thermal decomposition of calcium carbonate
Thermal decomposition
Calcium carbonate also reacts with _____ to make a _____, _____ and _____
Chemical equation for the reaction between calcium carbonate and sulfuric acid
The type of salt depends on the type of _____
Other carbonates that react with acids
Calcium oxide reacts with _____ to form _____
Chemical equation for the reaction between calcium oxide and water
Calcium hydroxide is an _____ which can be used to neutralize _____ in fields, and works much _____ than powdered limestone
Calcium hydroxide can also be used to test for _____ by making a solution called _____
If carbon dioxide is present, the limewater will turn _____, caused by the formation of _____
Chemical equation for the reaction between calcium hydroxide and carbon dioxide
Limestone can be _____ and _____ in a kiln with _____ to make _____
Cement can be mixed with _____ and _____ to make _____
Or cement can be mixed with _____ and _____ (water and gravel) to make _____
5 environmental issues caused by quarrying limestone
2 environmental issues caused by using limestone production
5 uses of limestone
2 good things about quarrying limestone
Advantages of limestone
Advantages of concrete
Disadvantages of limestone/concrete
Some _____ metals such as _____ are found in the earth as the metal itself
A metal _____ is a rock that contains _____ to make it _____ extracting the metal from it
In many cases the ore is an _____ of the metal
The main aluminium ore is called _____
The _____ of metal extraction can _____ over time and affect how _____ it would be to extract certain metals
A metal can be extracted from its ore by _____ or _____
Some ores may need to be _____ before extraction
Electrolysis can also be used to _____ the extracted metal
A metal can be extracted from its ore by reduction using _____
When an ore is reduced _____ is _____ from it
The position of the metal in the _____ determines whether it can be extracted by ______ with _____
Metals higher than _____ have to be extracted by _____ which is _____
Metals below _____ can be extracted by _____ using _____, for example _____
Metals that are more reactive than carbon have to be extracted using _____ of _____, such as _____
This is very expensive because it uses a lot of _____
While copper can be extracted using _____, it is very _____ and cannot _____ very well
So electrolysis is used to _____ it
Electrolysis is the _____ of a substance using _____
It needs a _____ to conduct the electricity, called the _____, often a _____ solution, such as _____, or a _____
The electrolyte has _____ which conduct the electricity
Electrons are _____ copper atoms at the _____, causing them to go into _____ as _____ ions
_____ ions near the _____ gain electrons and turn back into _____
The _____ are dropped at the _____ as a sludge, whilst _____ bond to the _____
Copper can also be extracted from _____ using a _____ reaction
More reactive metals react _____ than _____ metals
Placing a _____ metal into a solution of a _____ will mean that the _____ metal will _____ the _____ metal in the _____
The _____ metal bonds _____ to the _____ part of the compound and _____ the _____ metal
For example, _____ can be used to displace _____ from _____ solution
The supply of _____ ores is _____ so it is important to _____ as much as possible

 Hide known cards
Hide known cards