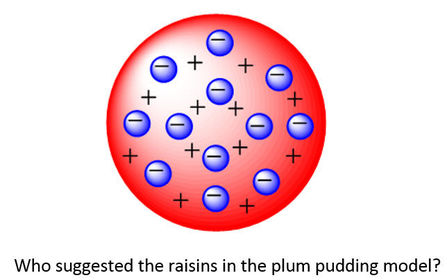
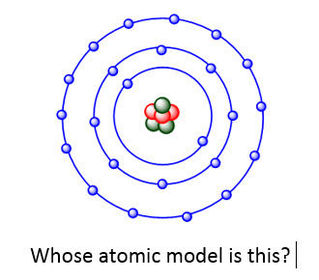
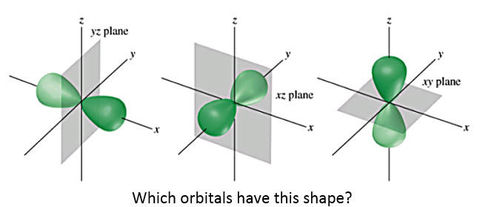
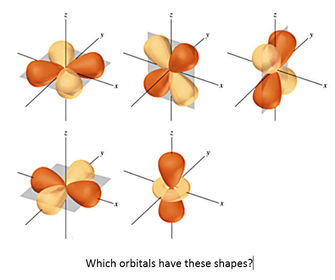
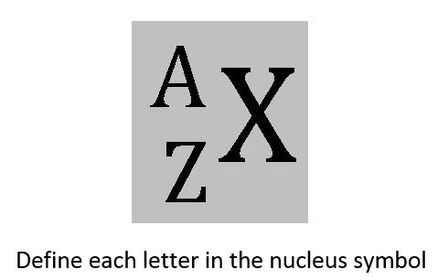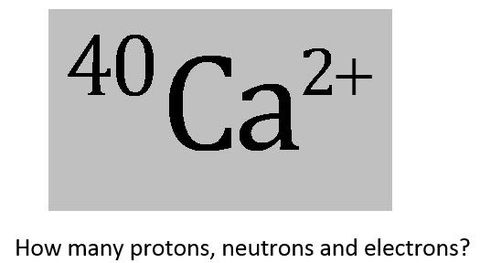University first year GeneralChemistry1 (Atomic Structure) Flashcards on Atomic structure, created by Chuleeporn Thanomsilp on 27/05/2014.
Pinned to
122
3
0
No tags specified
|
|
Created by Chuleeporn Thanomsilp
over 10 years ago
|
|
Close