A level (3.2 Physical Chemistry) Chemistry Fichas sobre Bond Enthalpies, creado por Yinka F el 05/03/2018.
Pineado a
0
0
0
Sin etiquetas

|
Creado por Yinka F
hace más de 6 años
|
|
Cerrar

|
Creado por Yinka F
hace más de 6 años
|
|

What is bond dissociation enthalpy?
Compare the energy required to break bonds with the energy released when bonds are made during an exothermic reaction
Compare the energy required to break bonds with the energy released when bonds are made during an endothermic reaction
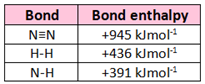
Nitrogen reacts with hydrogen to form ammonia in the reaction:
N₂ + 3H₂ --> 2NH₃
The energy needed to break all the bonds in N₂ and H = 2253kJmol⁻¹
The energy released when forming the bonds in NH₃ = 2346kJmol⁻¹
Is this reaction endothermic or exothermic?
What is the name for the difference between the energy absorbed and released?
Calculate the enthalpy change for the following reaction:
N₂ + 3H₂ --> 2NH₃

 Ocultar las fichas que te sabes
Ocultar las fichas que te sabes