Cerrar

1.Sketch the fcc and bcc unit cells. Which one has the higher atomic packing? (APF) Which one has the higher density?
Make a sketch of the cubic crystal plane with miller indices (¨6¨26)
(Prickarna är streck över siffran)
1.A cubic plane has the following axial intercepts: a = -2/3, b = 6, c = 1. What are the Miller
indices of this plane? Give also the normal direction to this plane!
1.Draw the following directions in a cubic unit cell: [00¨1 ], [113], [ ¨112], [ ¨52¨1 ].
(Prickarna är streck över kommande siffra)
1.Write down the Miller indices of all cubic face plans.
1.Make a sketch of a hcp unit cell.
1.How can you determinate if a crystal structure is a fcc or bcc type?
1. Make a sketch of the potential energy-versus-interatomic separation curves for a strongly and
a weakly bonded material.
1. Make sketches of all planes of the type {110}! (You may want to use more than one sketch.)
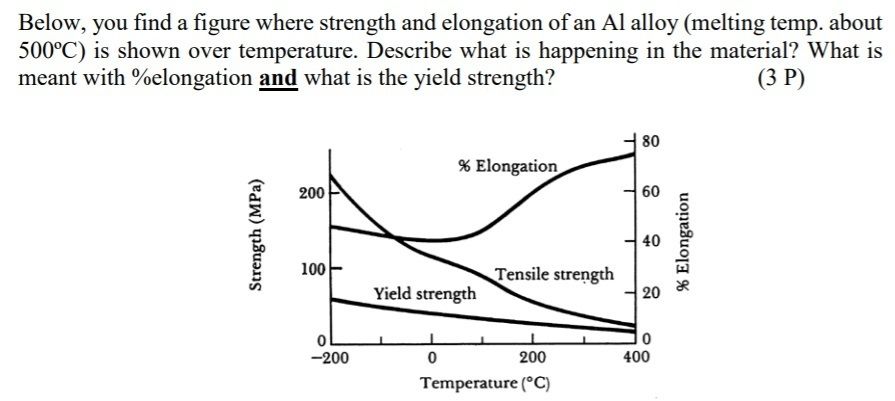
2. There are different methods of strengthen metal. Name them!
2. How can you strengthen a polymeric material?
2. You are supposed to deform both copper (Cu) and magnesium oxide (MgO. How are dislocations moving in these material and what type of problem may you encounter (especially in MgO)? describe in words or make a sketch for illustration.
2.Draw a stress-strain curve of a metal and explain in which regime of the curve you should deform/shape a metal!
Should a metal be shaped at room temperature or elevated temperature?
2. What is the strenghtening relate to/how does it work?
3.Make a sketch of an edge dislocation and explain/sketch how it's gliding.
3.What is required/what are prerequisites for diffusion to happen?
3. The diffusion coefficients for self-diffusion and carbon diffusion in α-iron at 500ºC are 3.0 10-21 m2/sec and 2.4 10-12 m2/sec, respectively. Explain the huge difference!
3. What is happening in carburization and why is it used?
3. In order for two components to have complete solid solubility in each other, they usually have to follow certain conditions. Name them!
3. Which hardening mechanisms can be achieved in an alloy (legering) with two elements that are totally soluble (löslig) in eachother?
3. Why is substitutional diffusion slower than interstitial diffusion? Explain briefly!
3. How can you distinguish between an edge and a screw dislocation? Describe briefly!
4. Draw cooling curves of a single component and a binary system!
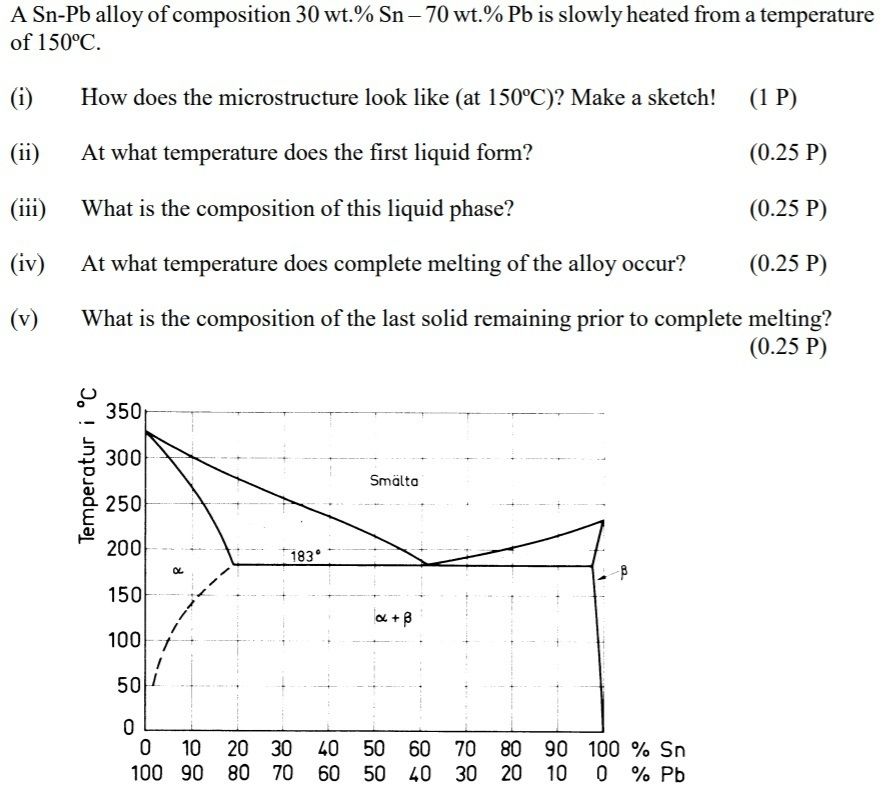
4. Depending on temperature, pure Fe occurs in different crystal structures. Give the name of the different types of Fe, their crystal structure, their temperature range of existence, and
the highest amount of carbon they may contain. The Fe-C phase diagram is given below!
4. What is the difference between a eutectic and a eutectoid reaction? Explain and give
examples for both reactions.
4.For a Fe – 0.5 wt% C alloy at a temperature at 725ºC, determine the phase amounts of
primary ferrite, total ferrite and of the cementite phase.
4.Make sketches of the microstructure of the Fe – 0.5 wt% C alloy at 750 and 650ºC!
5.What is a martensitic transformation? Explain! Where to find martensite in the
Fe-C phase diagram?
5. Explain the difference between homogeneous and heterogeneous nucleation and state which one is more advantageous and why!
5. You have a lamellar structure, for example pearlite. How can you determine if the
microstructure was achieved at higher or at lower temperature?
6. Give estimates of the used energy over life time of a civil airplane and a vacuum cleaner.
Motivate your answer!
6. With respect to recyclability compare metals and polymers. Recyclability of which of the materials is more economic and why? For comparison, how would you rank fiberreinforced polymers? Motivate!
6. The total energy use of a product is given by 4 different energies. Name them!
6. Briefly assess energy-use-over-life for a mobile phone!
7.Explain – in words and with a sketch – the difference in electrical conductivity (or electron concentration) upon temperature of an intrinsic semiconductor and an extrinsic semiconductor
7. Sketch the electron energy band structure of a p-type semiconductor and explain how
conductivity can be achieved.
7. What are the major charge carriers in
(i) an intrinsic semiconductor,
(ii) an n-type
semiconductor,
(iii) a p-type semiconductor, and a
(iv) metal?
7. Ceramic are usually bad conductors or in fact insulators. Describe a case where ceramicmaterial can be an excellent electrical conductor. What is required to achieve that?
7. Matthiesen’s rule describes what is affecting conductivity in metals? Name the effects!
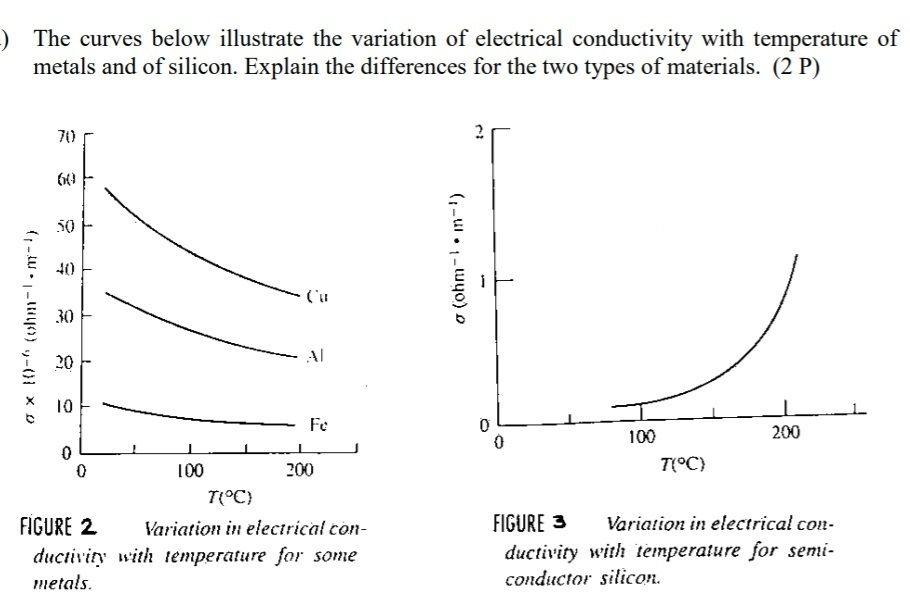
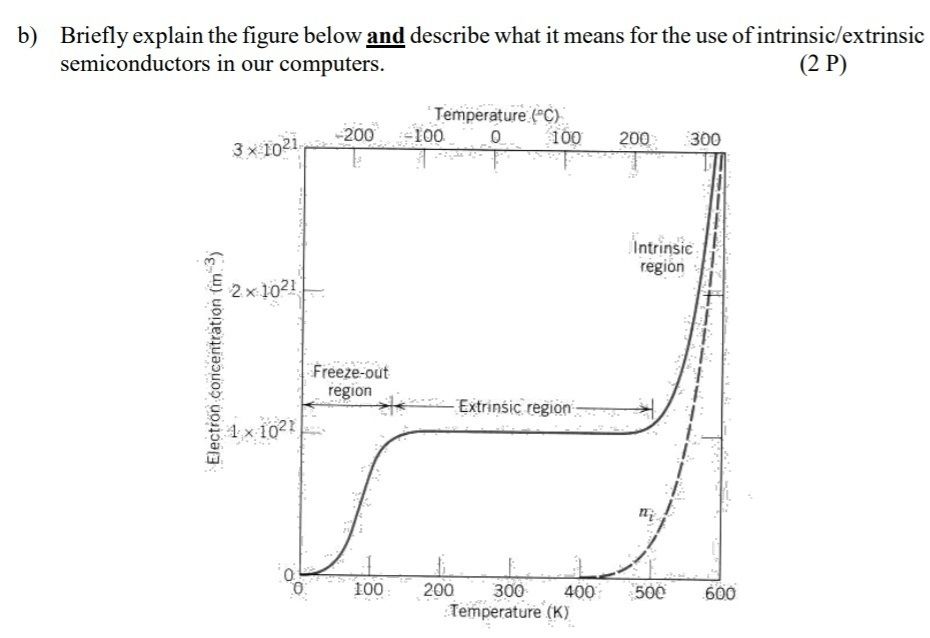
7. Knowing the electron energy band structure, why can you predict if a material is a good or bad thermal conductor?
7. Make sketches of the electron energy band structure of a p-type and an n-type
semiconductor (incl. defect level) and briefly explain for both cases how
conductivity can be achieved.
8. How is heat transported? Explain!
8. Make a sketch of the potential energy versus interatomic separation curve for a strongly bonded and a weakly bonded material.
8. Why don’t you burn yourself at your steel sink when you are doing the dishes? Explain briefly!
8. Why are ceramics sensitive to thermal shock? Explain (case to be chosen).
8. What is thermal expansion and how is it related to bonding? Explain!
Explain ball bonding and name the wire which has to be used!
Explain what is meant with electromigration!
What are direct and expanded contacts? Explain advantages!
Describe ball bonding!
9. What is epitaxy? Explain briefly!
What is happening when you join a p-type and an n-type semiconductor? Explain!
What is happening in case of forward bias and reverse bias? Explain briefly!

 Ocultar las fichas que te sabes
Ocultar las fichas que te sabes