Chemistry Fichas sobre 2. Acids & Bases, creado por John Rivada el 19/04/2019.
Pineado a
3
1
0
Sin etiquetas
|
|
Creado por John Rivada
hace más de 5 años
|
|
Cerrar
|
|
Creado por John Rivada
hace más de 5 años
|
|

What is a Bronsted acid?
What is a Bronsted base?
What is the equation for pka/ka?
Define amphoteric
What must the Ka value be for an acid to be completely dissociated?
Is the following reaction capable of happening? Why or why not?
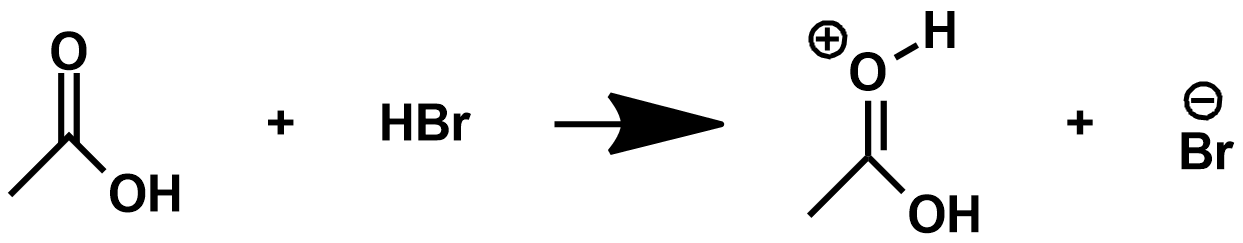
What is a zwitterion?
If acid is weak, is pH high or low?
If acid is strong, is pH high or low?
If acid is strong, is pka high or low?
If acid is weak, is pka high or low?
If base is weak, is pH high or low?
If base is strong, is pH high or low?
If base is strong, is pka high or low?
If base is weak, is pka high or low?
Define pka and pkb
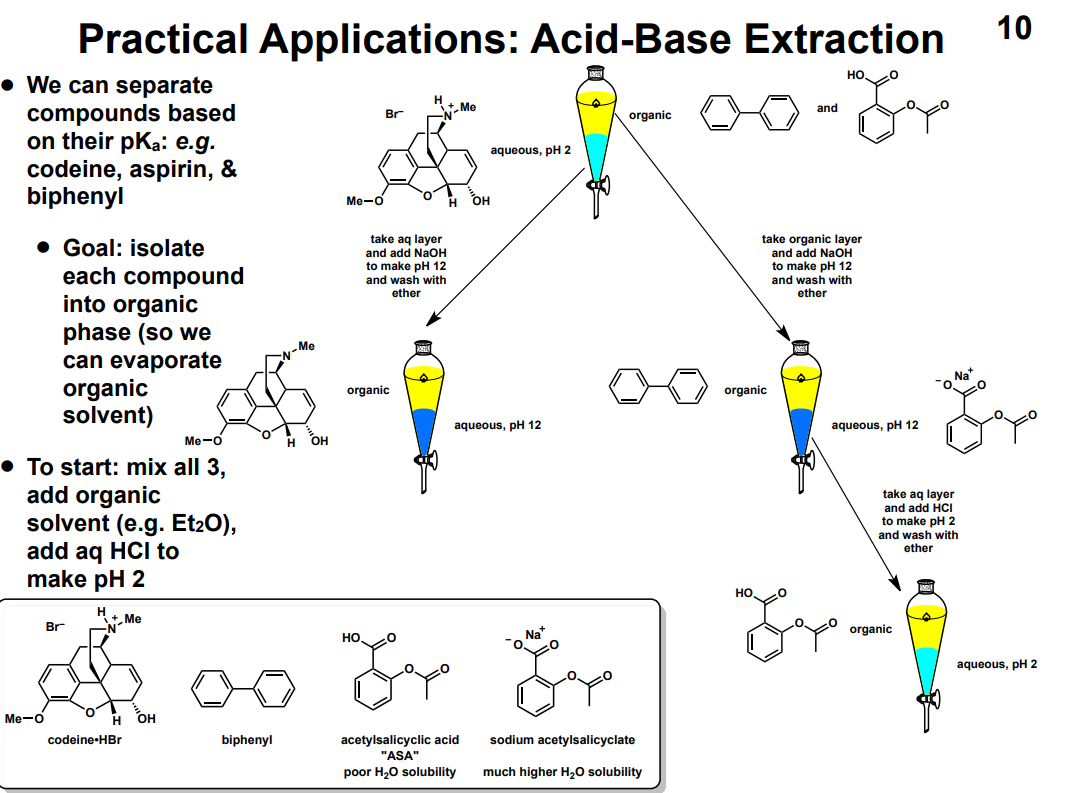
Conjugate base is MORE stable than acid: strong or weak acid?
Conjugate base is LESS stable than acid: strong or weak acid?
What makes an anion/conjugate base stable?
Conjugate acid is MORE stable than base: strong or weak base?
Conjugate acid is LESS stable than base: strong or weak base?
What makes a conjugate acid stable?
Examples of HARD
Common HARD reagents
What is the full name and chemical structure for DIBAL
What is its main use?
Examples of SOFT
Common SOFT reagents
Calculate Keq of the above equation. Is it a complete reaction?
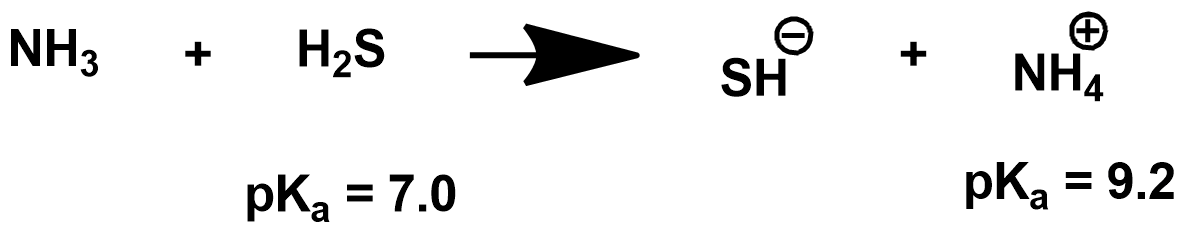
How do you calculate log Keq? Or just Keq?
Why is that H3O+ is strongest acid in water?
True or false: do solvents affect pKa?
Pka?
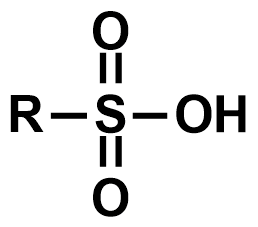
Pka?
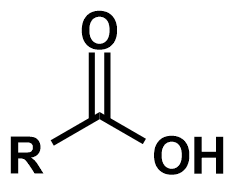
Pka?
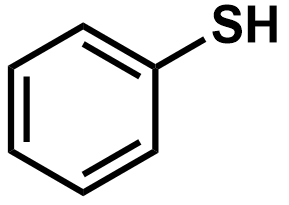
Pka?
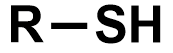
Pka?
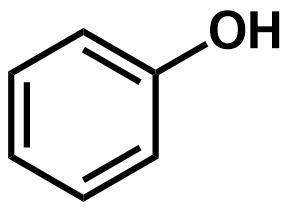
Pka?
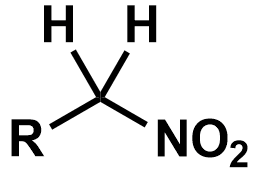
Pka?
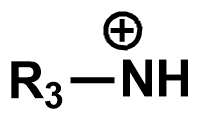
Pka?
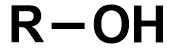
Pka?
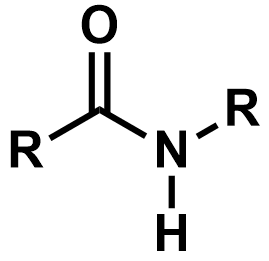
Pka?
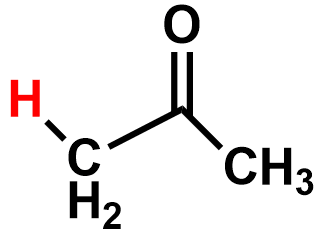
Pka?
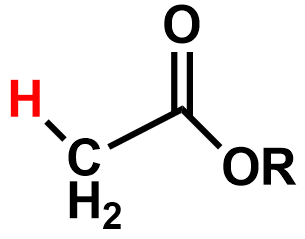
Pka?

Pka?

Pka?
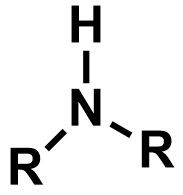
Pka of alkanes?
Pkb range of alkyl- and aryllithiums?
Pkb of LDA?
Does an EWG make a compound more or less acidic?
Does an EDG make a compound more or less acidic?
Does hybridization make a compound more or less acidic?
Ie is an alkyne more or less basic than an alkane?
What is good about LDA
Mechanism of LDA formation?
Are alkali metal hydrides good nucleophiles? Good bases?
PKb of H-?
ie Pka of H2?
Pkb >= pka - 6
Equilibrium favours which direction?
What is an amidine? (chemical structure)

 Ocultar las fichas que te sabes
Ocultar las fichas que te sabes