Cerrar

Concepts to understand
Biochemistry is the chemistry of life
What are the properties of life in the context of biochemistry?
• Incredible complexity and organization
Information (genetical, structural) => __ entropy
Biomolecules - organelles — cells — tissues — organs — organisms
Homeostasis (body temperature, salts, voltage potentials, ...)
Metabolism (anabolism, catabolism, pathways)
Responsiveness to the environment
Growth & Reproduction
Adaptation, including genetically
The principles of biochemistry ar common to ___ living beings.
Biochemistry VS. Organic Chemistry
-Very __ range of temperatures
-Narrow range of pH
-Atmospheric pressure
-Water is the major ____ (versus a wide variety in organic chemistry)
-Carbon-based (limited choices of elements & molecules)
How does LIFE find solutions? ____
Life is ___ based
Why C?
• Four ___ bonds => diversity of 3D structures
• Stable double, triple bonds
• Forms/breaks bonds with _, _, & _
• Reversible oxidation/reduction
C-based molecules active under the ____ conditions
Alternatives to C?
B : 3 electrons; not stable
5 electrons; N-N-N => explosives
Si: Large radius => Si-Si is a weak bond
P : Not stable
Other elements of life
• Oxygen, hydrogen, nitrogen, phosphorus, sulfur are major elements
In addition to homo- (C-C), form heteronuclear bonds (C-O)
These covalent bonds can break and form
Several other elements are in small (trace) quantities
Examples: Ca2+ Mg2+ Fe2+ & Fe3+
Water is an essential solvent and ___
-->50-95 % (by body weight)
-->Unique properties: small size, chemical stability, low viscosity, polarity, melting & boiling points, ...
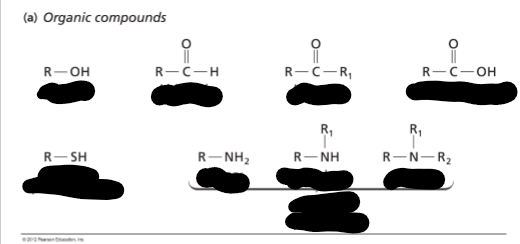
Basic Organic Compounds encountered in biochemistry
Four classes of biological macromolecules
WHat are the functions of each?
l. Carbohydrates = ____
ll. Lipids = ____
Ill. Nucleic Acids = ____
IV. Proteins = ____
Most of the biological macromolecules, except for lipids, are ___
Polymers are formed from like monomers
Depending on the polymer, different types of ___ bonds formed
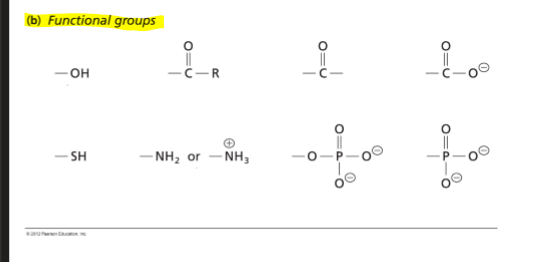
Functional groups (FG)
-can be distinguished in all four types of biological macromolecules (same FG can be a part of a wide variety of "life" molecules)
FG: a group of atoms that demonstrate characteristic reactions
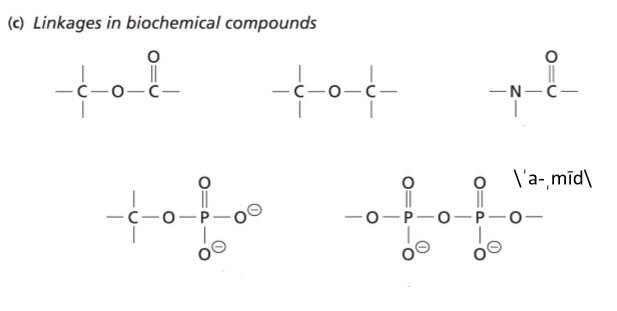
Biomolecules (biopolymers) are held together by linkages
Carbohydrates = Saccharides = ___
Example = Glucose
FG's = hydroxy & carbonyl
Glucose interconverts b/w alpha & beta forms and is in equilibrium in solution
Monosaccharides (single sugars)
Glucose, Fructose, Galactose
Disaccharides (2 sugars)
Sucrose = table sugar
Lactose
Maltose
Oligosaccharides
-consist of a few (3-10) monosaccharides
Polysaccharides
-are long polymers
What happens when you digest sugar?
-Ether bond breaks with the addition of water (____ reaction).
-Enzyme plays a key role.
Sugars/Carbohydrates - Function
• Energy source — e.g. glucose
• Structure — e.g. ___
• Part of another biomolecule:
v/ Nucleotides contain a sugar moiety
v/ Glycoproteins: ___ with covalently attached a carbohydrate
Glycolipids: lipids with ____ covalently attached
These complex molecules will be discussed later in detail
Protein is a polymer of ___ ___
• a-amino acids are the building blocks
• Peptides is < __ amino acids
• ____ is >50 amino acids
• A protein is one or more polypeptides
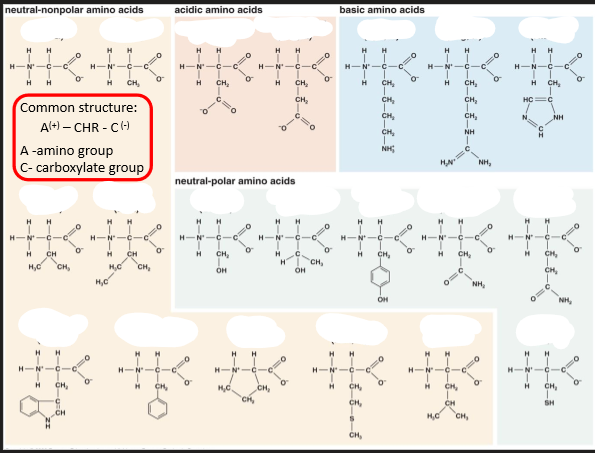
Amino Acids
• a-amino acids are building blocks of proteins
• In peptides, amino acids are covalently attached via ___ bonds
There are 20 standard a-amino acids in proteins
• Properties of amino acids and proteins are determined by the properties of the ___ ___ of the amino acids
• Amino acids can be grouped depending on the chemical properties of
their side chain: charge, polarity, acidity, interaction with water, ...
• Polypeptides are folded to form a functional 3D structure. In water hydrophobic residues are buried ___. -->Membrane proteins - opposite
Dehydration reaction forms the peptide (amide) bond
Nucleic Acids (DNA, RNA)
Nucleic acid is a polymer of ___ monomers
Nucleotides are formed from three functional groups:
1. Phosphate group (P)
2. A 5-carbon sugar: (R) Ribose, (D) Deoxyribose
3. Nitrogenous base: (A) Adenine, (G) Guanine, (C) Cytosine, (T) Thymine, (U) Uracil
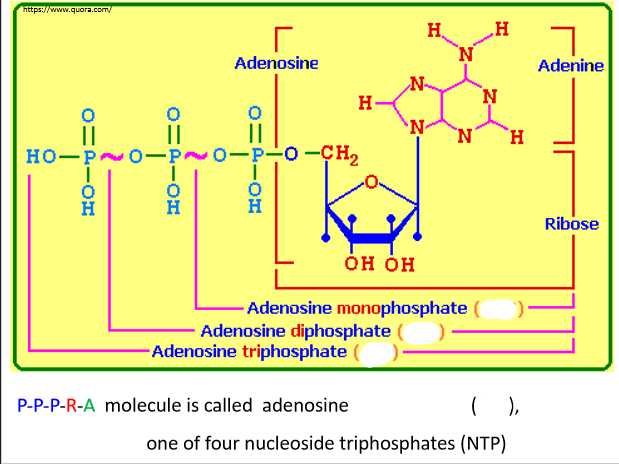
Nucleic Acid Synthesis
NTPs (nucleoside triphosphates) are ___ building blocks
dNTPS (deoxynucleoside triphophates) are ___ building blocks
LIPIDS
Lipid molecules contain relatively large ___ fragments
___ : do not dissolve in water but do dissolve in non-polar solvents
Do NOT form ____
Simplest lipids are ___ ___
FAs consist of a terminal carboxyl group (___) and hydrocarbon tail or chain (___).
Glycerophospholipid
-Amphiphilic
-Form bilayers
Triglyceride (triacylglycerol, TAG, TG) is an ester-linked glycerol & 3 FAs
-FAt found in the ___
-Excessive calories are saved as __ ___ in the form of triglyceride
-Skin oil
-High levels in the blood may cause stroke, heart diseases, pancreatits, ...
Waxes
Steroids

 Ocultar las fichas que te sabes
Ocultar las fichas que te sabes