GCSE Chemistry Fichas sobre Chemistry 1 Atoms, Elements and compounds Core GCSE, creado por Chloe Roberts el 21/04/2014.
Pineado a
197
5
0
|
|
Creado por Chloe Roberts
hace más de 10 años
|
|
Cerrar
|
|
Creado por Chloe Roberts
hace más de 10 años
|
|

What does each of these substances have in common:
CH4, HCl, MgO, Ne, 02, SO2
What are the names and numbers of the particles in an atom of sodium (Atomic number 11, mass number 23)
What determines the order of the elements in the periodic table
Explain why boron and aluminium are both in the same group on the periodic table
Name the type of bond in a metal and non metal bond
Name the type of bond in a bond containing two non metals
Explain what happens to the atoms when a sodium atom reacts with a chlorine atom
Calcium carbonate decomposes when heated to produce calcium oxide and carbon dioxide. 20.0g of calcium carbonate produce 11.2 of calcium oxide. What mass of carbon dioxide would be produced. Explain How
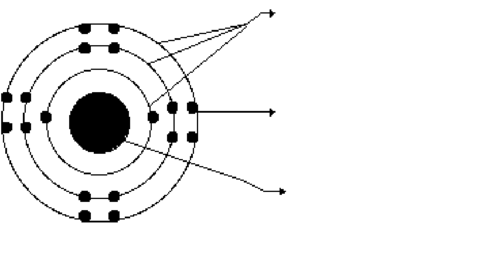
What does each of these substances have in common:
Ca, H2, Ne, O2, SO2

 Ocultar las fichas que te sabes
Ocultar las fichas que te sabes