16407430
Introduction to Bioenergetics
Description
No tags specified
Flashcards by Alice Hathaway, updated more than 1 year ago
More
Less
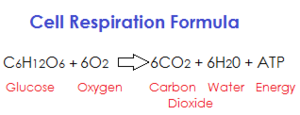
|
Created by Alice Hathaway
over 5 years ago
|
|
Resource summary
| Question | Answer |
| Thermodynamics | Cells appear to violate through high order 1st law = energy cannot be creates or destroyed 2nd law = in spontaneous process processes, entropy of universe increases |
| 1st law impact | Change in internal energy = heat energy absorbed - work done by system Places upper limit on energy cost - energy store less than or equal to energy coming in to system |
| 2nd law impact | Entropy = degree of disorder At equilibrium, no change in entropy When all reach equilibrium, entropy death Entropy hard to measure experimentally |
| Gibbs Free Energy | Free energy = change in energy - temp x change in entropy |
| Impact of Gibbs | For spontaneous reactions, change <0 - through high temperature/ large increase in entropy/ highly exothermic If >0. not spontaneous hence energy needed |
| ΔG' | Standard Gibbs energy change for reaction at pH 7 Can measure by measuring equilibrium constant ΔG' = -RT ln [product]/[reactant] |
| State function | Change processes same regardless of pathways |
| Couple reactions | Can do this to make ΔG <0 Reaction thermodynamically unfavoured coupled to one that is to drive reaction |
| ATP is driving force | Coupled to reactions to make thermodynamically favourable ΔG' = -30.4kjmol-1 Energy of hydrolysis depends on concentration of reactants nadproducts and concentration of Mg2+ and Ca2+ ~50jkmol-1 under typical conditions |
| Calculating ΔG value | -30.5 + RT ln [ADP][Pi][H+]/[ATP] |
| Why exothermic? | Phosphate and ADP more resonance stabilisation than ATP Electrostatic repulsion in phosphate More water can bind ADP and Pi than ATP |
| Phosphorylation potential | phosphorylation potential between biologically important phosphorylated molecules Can be phosphorylated by those with lower potentials Phosphorylates those with higher potentials Phosphocreatine phosphorylates ADP - energy store to safeguard ATP |
| Measuring ATP changes | Turnover of 0.5kj/min in exercise ATP buffered by phosphocreatine in many mammalian tissues In high energy demand/ lack of oxygen, Pcr decrease in concentration prior to depletion of ATP |
| Coupling ATP hydrolysis to chemical reactions | Phosphorylates glucose so can be broken down ATP used to build peptides Join 2 nucleic acids to synthesise DNA ATP changes equilibrium constant by 10^5 - shifted to form product |
| Activated carriers | |
| Reduction and oxidation | In reduction, NAD+ receives H+ and 2e NADP+/NADPH used in biosynthesis Phosphate group acts as tag to allow recognition of redox system by enzymes Can achieve 2 redox potentials - ATP synthesis and metabolites |
| ACetyl Co A | Reactive sulphur bonds CoA needed to catalyse acylation Hydrolysis G'=-31.5kjmol-1. Activated acyl group Universal acyl group carrier e.g. fatty acids |
| Activated Coenzymes | Drive thermodynamically unfavourable reactions All contain adenine base - may have been used as reaction motif by early RNA catalysts |
Want to create your own Flashcards for free with GoConqr? Learn more.
