
Which is the mass number and which is the atomic number?
Is the top or along the edge the period or the group?
Periodicity (repeating trends in properties of elements) need to know about
Periodic trend in electron configuration
Advantages of groups 1-7 and 0 (old way) and not 1-18
First ionisation energy
Ionisation energy
Factors affecting attraction and therefore ionisation energy- Atomic Radius
Factors affecting attraction and therefore ionisation energy- Nuclear charge
Factors affecting attraction and therefore ionisation energy- Electron shielding
Second ionisation energy
Describe the difference between the size of first and second isonisation energy e.g. of helium
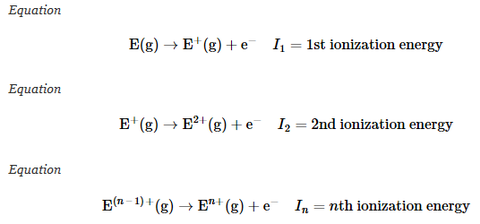
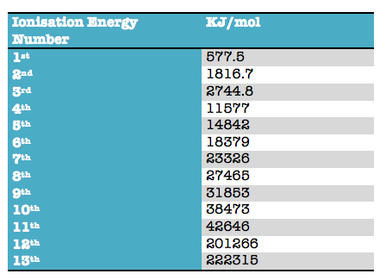
Describe the successive ionisation energy graph of sodium
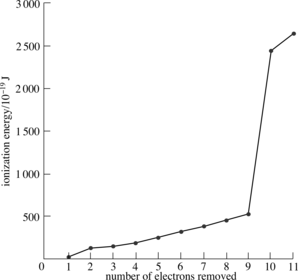
Successive ionisation energies allow predictions to be made about:
From the first 4 ionisation energies identify the element from period 3
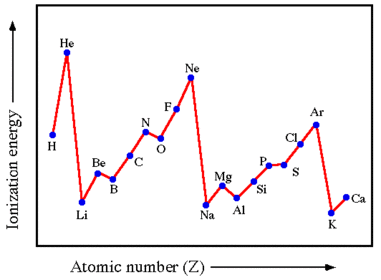
Key patterns with first ionisation energies
Why does 1st ionisation energy show an increase across a period?
Why does 1st ionisation energy show a decrease down a group?
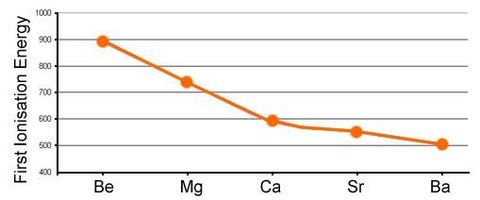
Why does nuclear charge increase but 1st ionisation energy decrease?
Across period 2 which elements experience significant falls in the ionisation energy meaning there is not a steady increase?
Why is there a decrease in 1st ionisation energy from beryllium to boron?
Why is there a decrease in 1st ionisation energy between nitrogen and oxygen?
What are metalloids/semi-metals?
Do all metals have the same properties?
How does conduction take place?
The structure of metal-metal bonding
Role of cations and electrons within the metallic structure
Properties of metallic bonding
Electrical conductivity in metallic bonding
Melting and boiling points of metallic bonding

Solubility of metallic bonding
Non metal and non-metals
Which non-metals do not have simple molecular structures?
Carbon (in it's diamond form) and silicion have a giant covalent lattice structure, but what shape structure do they form?
Melting and boiling points of giant covalent lattices

Solubility of giant covalent lattices
Electrical conductivity of giant covalent lattices
Graphene and graphite
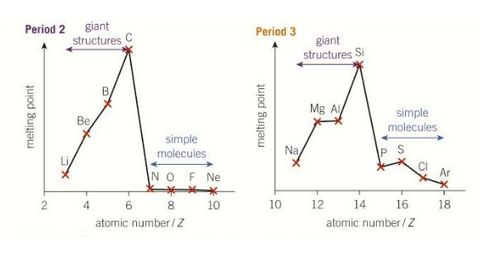
Periodic trends in melting points- group 2 and 3
Why is the periodic trends in melting point across periods 2 and 3 as they are?

 Hide known cards
Hide known cards
