A level Chemistry (3.2 Physical Chemistry) FlashCards sobre Hess's Law, criado por Yinka F em 05-03-2018.
Pin adicionado em
0
0
0
Sem etiquetas

|
Criado por Yinka F
mais de 6 anos atrás
|
|
Fechar

|
Criado por Yinka F
mais de 6 anos atrás
|
|

What does Hess's Law state?
TRUE OR FALSE:
The enthalpy change of formation for all elements is zero
Describe the Hess cycle used to calculate the enthalpy change of reaction for the reaction below, given the enthalpies of formation
SO₂ + 2H₂S --> 3S + 2H₂O
Describe the Hess cycle used to calculate the enthalpy change of formation for ethanol, given the enthalpies of combustion
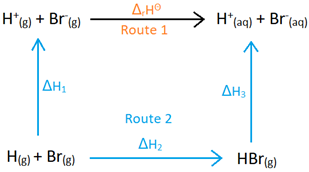
Using this Hess diagram, describe the calculations needed to calculate the enthalpy change of reaction for the following reaction

 Ocultar acertos
Ocultar acertos