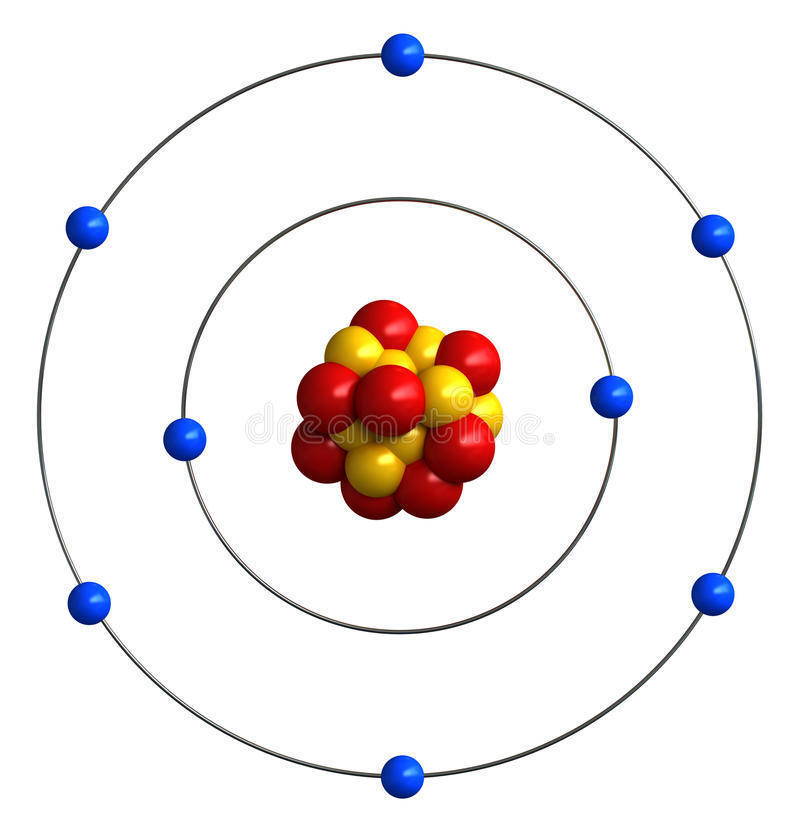
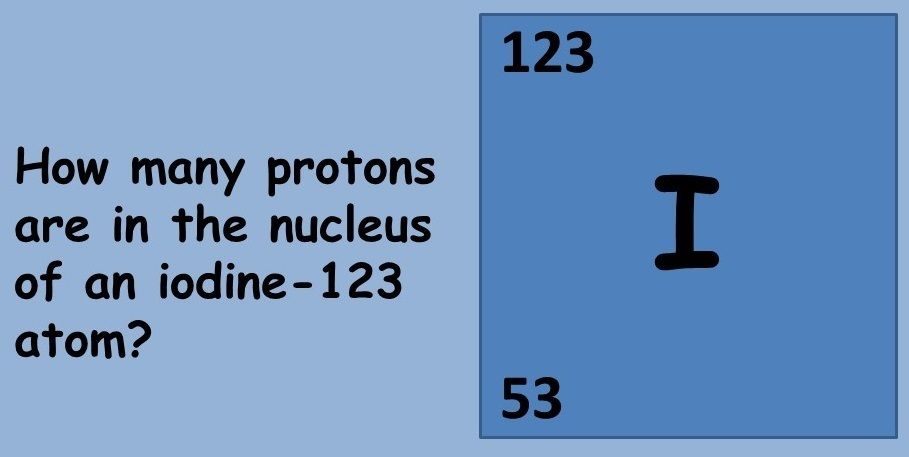
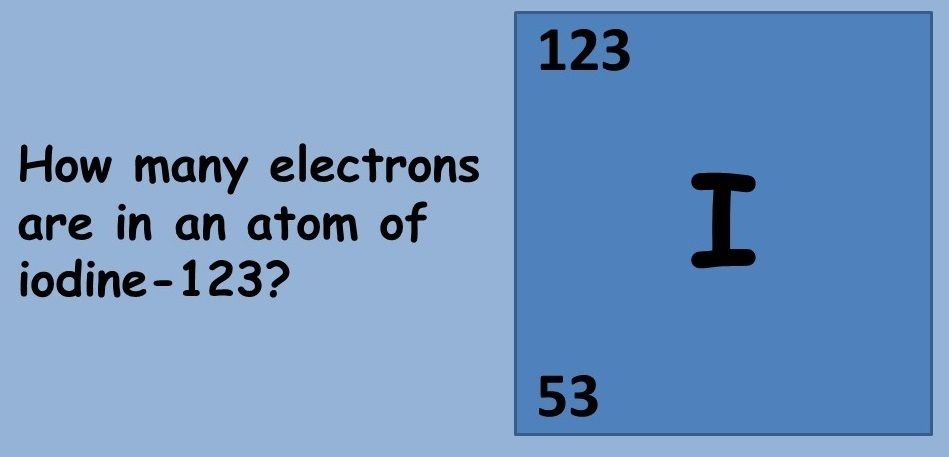
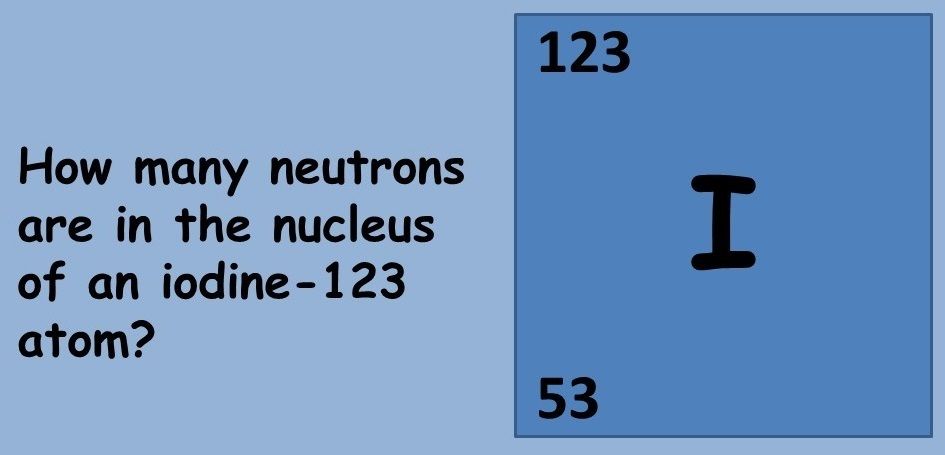
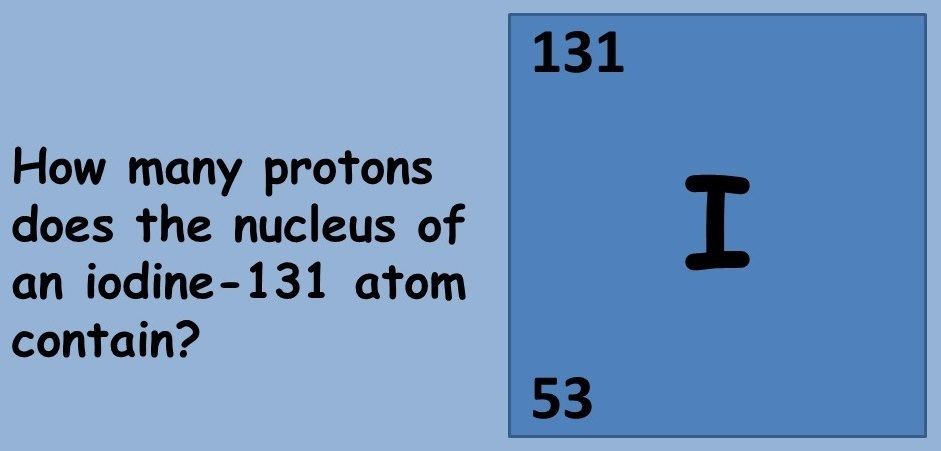
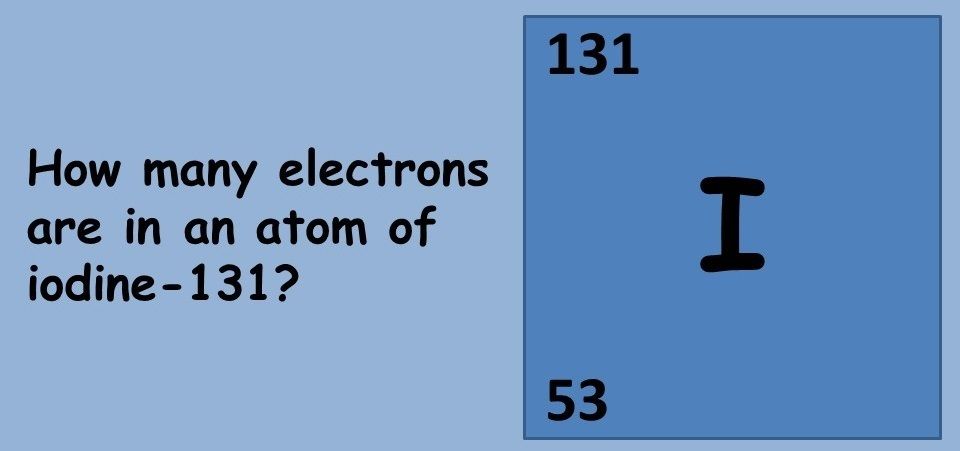
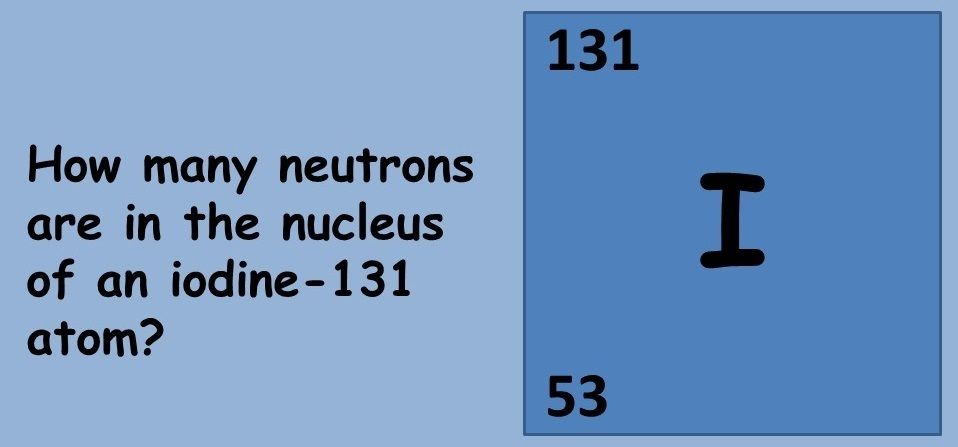
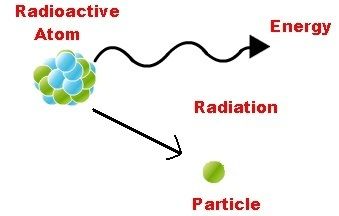
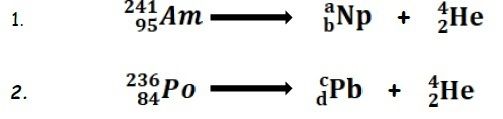
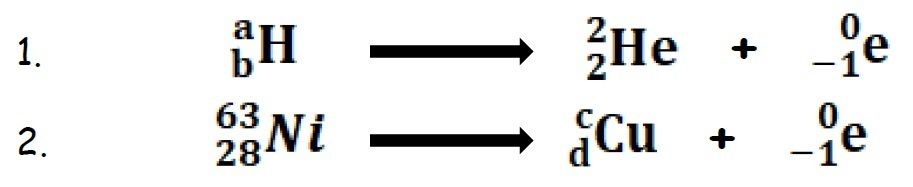
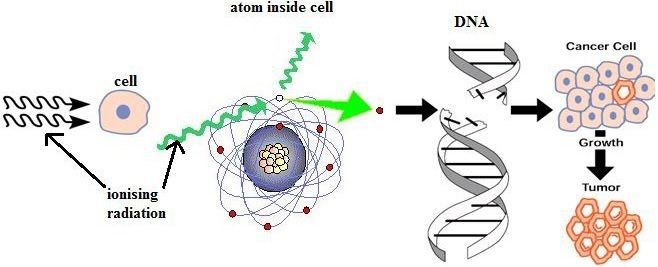
This topic covers the structure of the nuclear atom and its representation using atomic notation. It covers the spontaneous nature of nuclear decay and the nature of alpha, beta and gamma radiation. Learners will produce and balance nuclear equations for radioactive decay.
Pin adicionado em
310
0
0
Sem etiquetas
|
Criado por Mr S Lee
mais de 6 anos atrás
|
|
Fechar