FlashCards sobre nonspontaneous reaction, criado por Alvaro Vargas Calero em 02-05-2018.
Pin adicionado em
57
0
0
Sem etiquetas
|
|
Criado por Alvaro Vargas Calero
mais de 6 anos atrás
|
|
Fechar
|
|
Criado por Alvaro Vargas Calero
mais de 6 anos atrás
|
|

Nonspontaneous reaction
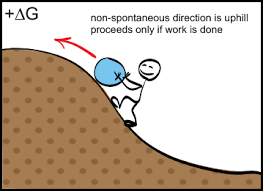
Out atmosphere is composed primarily of a mixture of nitrogen and oxygen gases. One could write an equation showing these gases undergoing a chemical reaction to form nitrogen monoxide.
Fortunately, this reaction is nonspontaneous at normal temperatures and pressures. It is a highly endothermic reaction with a slightly positive entropy change (ΔS) . However, nitrogen monoxide is capable of being produced at very high temperatures, and this reaction has been observed to occur as a result of lightning strikes.

 Ocultar acertos
Ocultar acertos