Chemistry FlashCards sobre 4. Chemoselectivity, criado por John Rivada em 20-04-2019.
Pin adicionado em
3
1
0
Sem etiquetas
|
|
Criado por John Rivada
mais de 5 anos atrás
|
|
Fechar
|
|
Criado por John Rivada
mais de 5 anos atrás
|
|

What are the three types of selectivity?
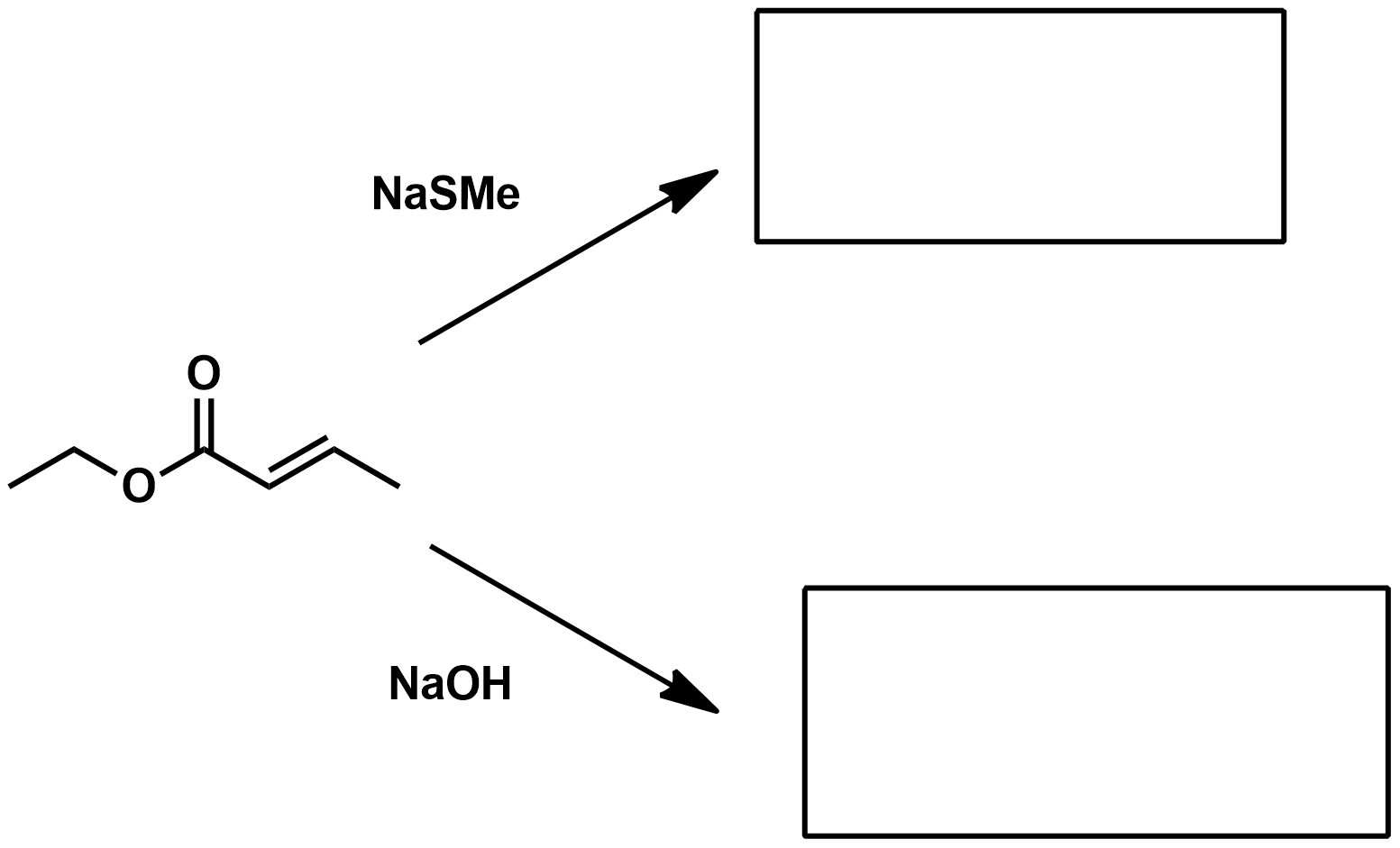
What is the reactivity scale towards nucleophiles?
Oxidation vs. Reduction
What are the 4 classes of reducing agents?
What are good reducing agents?
Which is the harsher reducing agent of the two:
NaBH4 or LiAlH4
Which of the following is the mildest and which is the strongest reducing agent:
LiAlH4, DIBAL, LiBH4
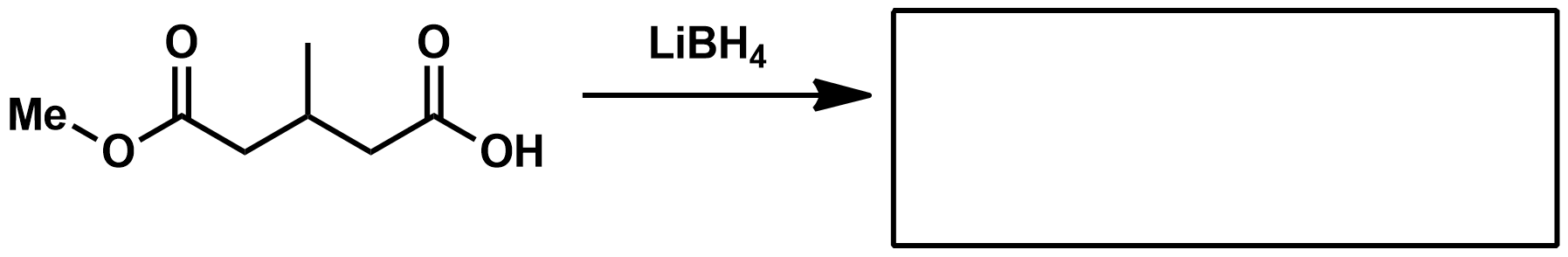
Using a reducing agent as the reagent, how can you make a 1° alcohol?
Using a reducing agent as the reagent, how can you make a 2° alcohol?
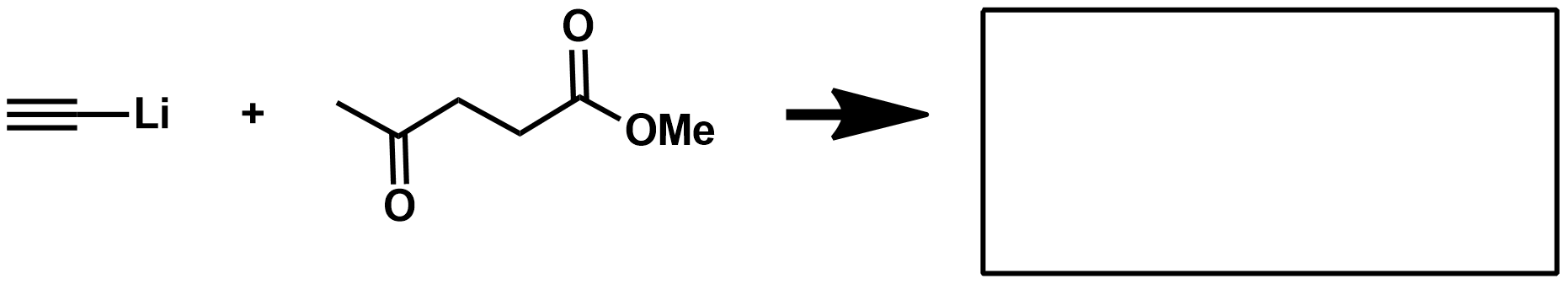
Mechanism for Aldehyde/Ketone reduction?
Mechanism for Ester reduction?
Mechanism for Amide reduction?
Using a reducing agent as the reagent, how can you make an amine?
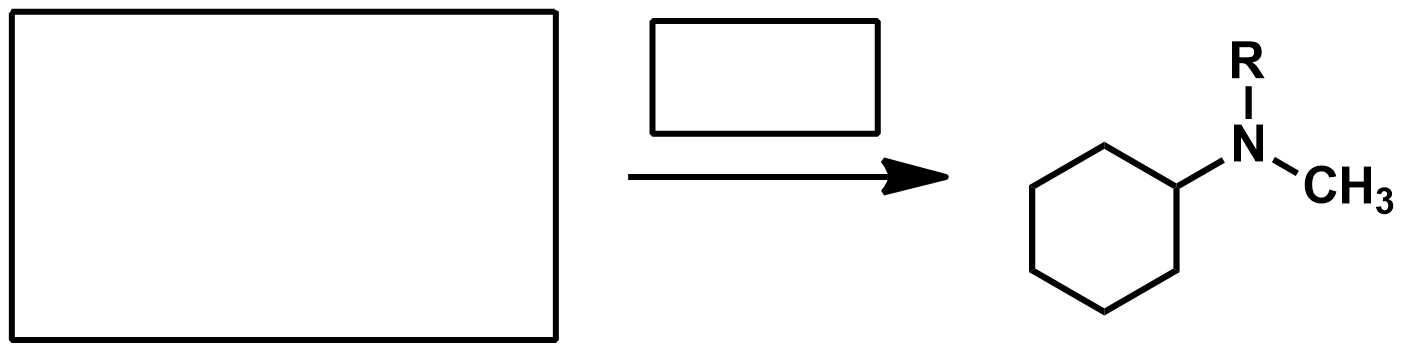
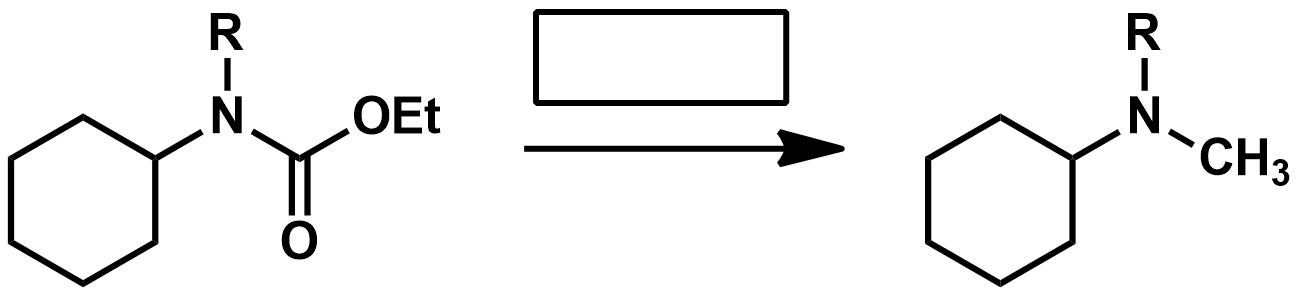
What should I use if I have a compound that has an eseter and a ketone and want to reduce both to alcohols?
LiAlH4 or NaBH4?
What should I use if I have a compound that has an eseter and a ketone and want to reduce just the ketone?
LiAlH4 or NaBH4?
Slide 18
*Left aldehyde is supposed to be carboxylic acid
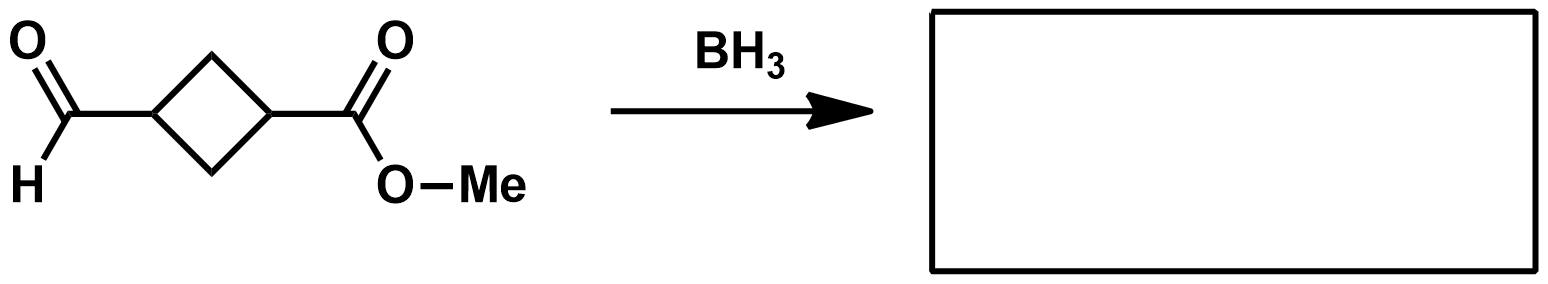
What's so good about BH3 as a reducing agent?
Difference between NaBH3 and NaCNBh3
What is the difference between hydrogenation and hydrogenolysis?
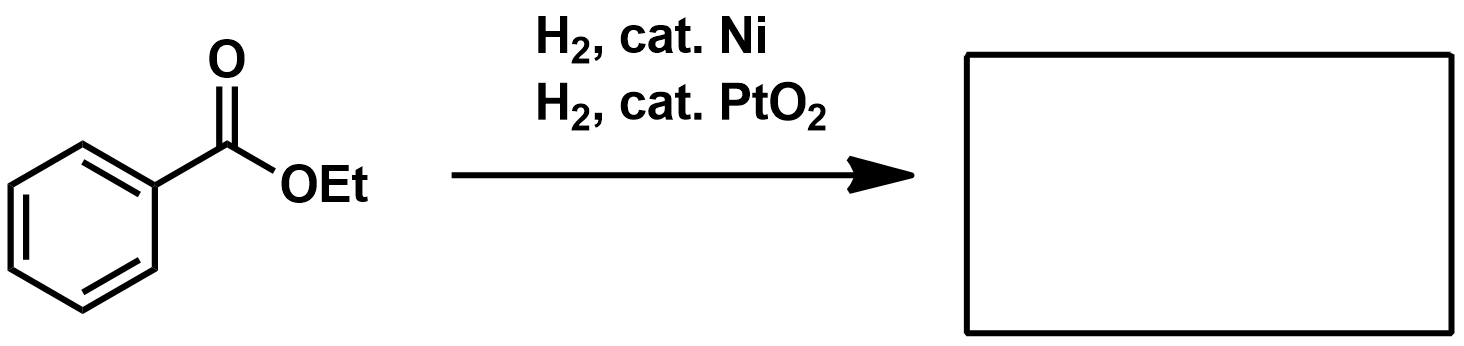
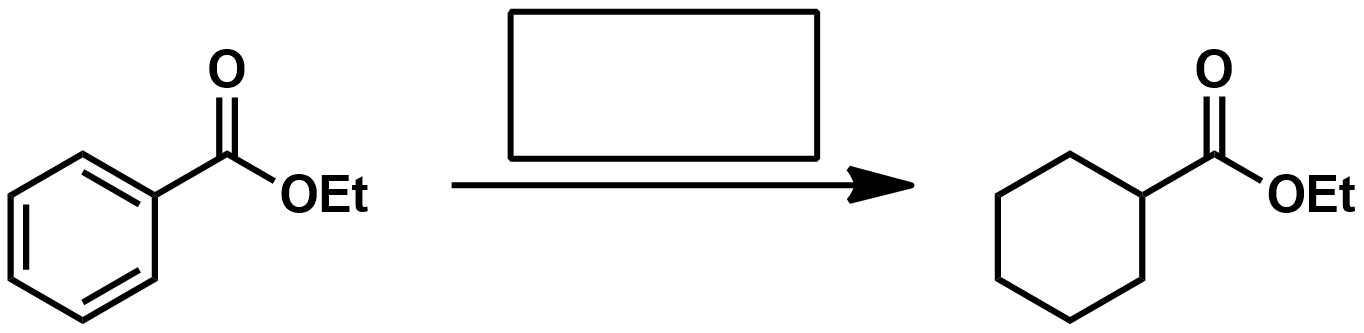
If your substrate is a benzyl amine or ether, what kind of metal should you use for hydrogenation?
If your substrate is an alkene, what kind of metal should you use for hydrogenation?
If your substrate is an aromatic ring what kind of metal should you use for hydrogenation?
Fill in the reagents
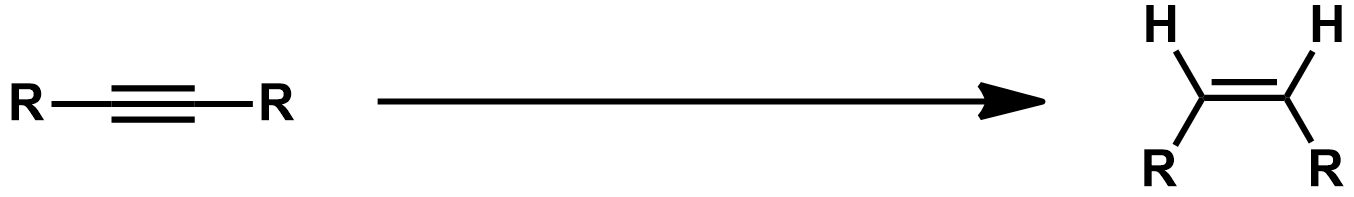
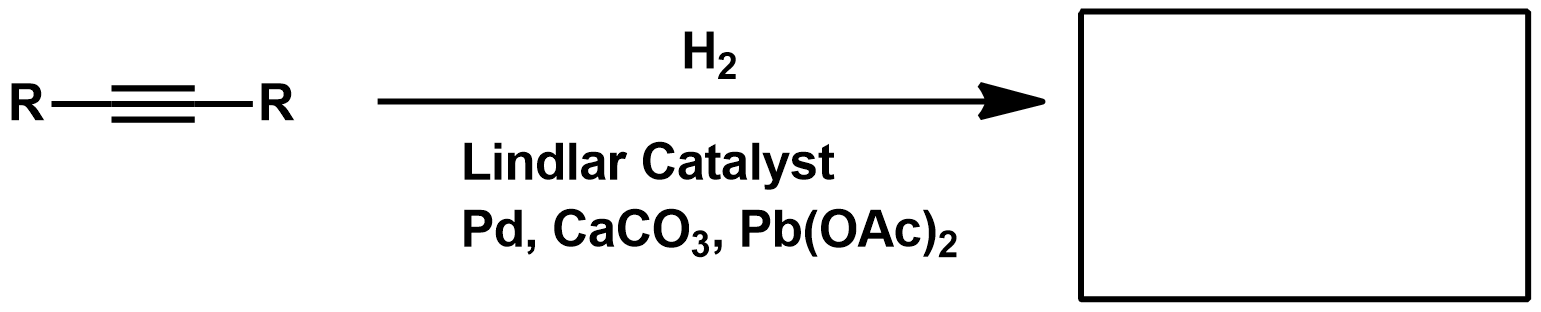
Examples of Lindlar Catalysts
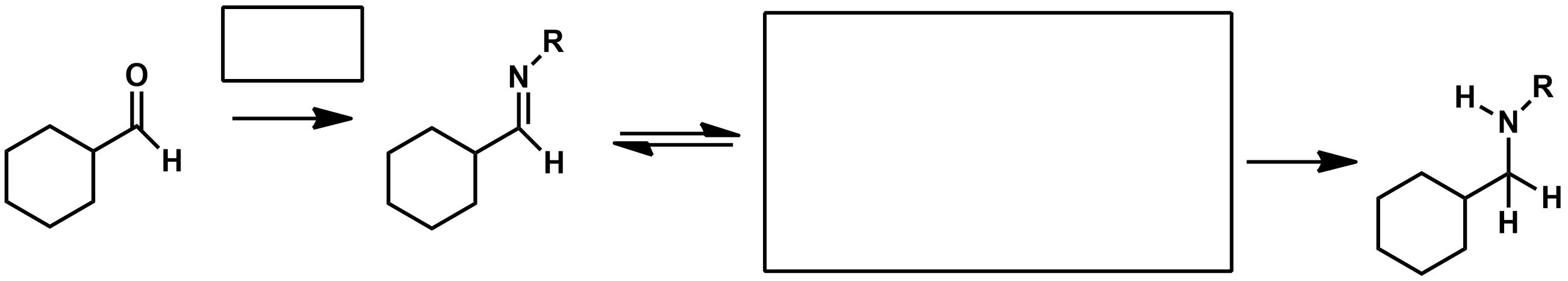
Using a reducing agent as the reagent , how can I turn an imine into an amine?
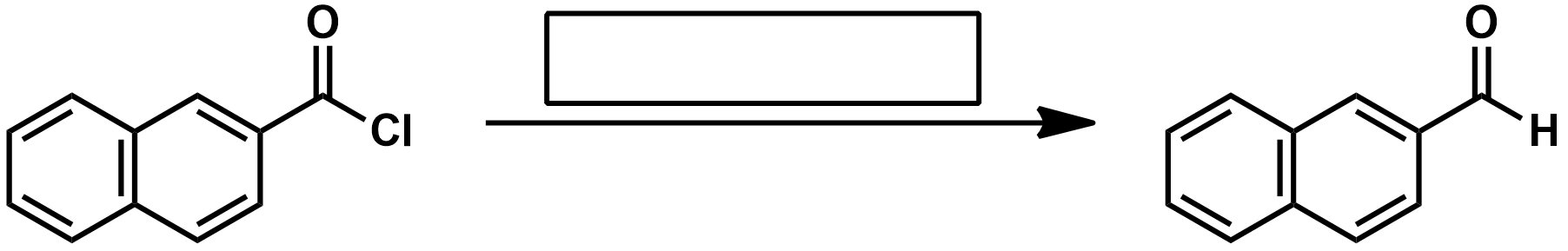
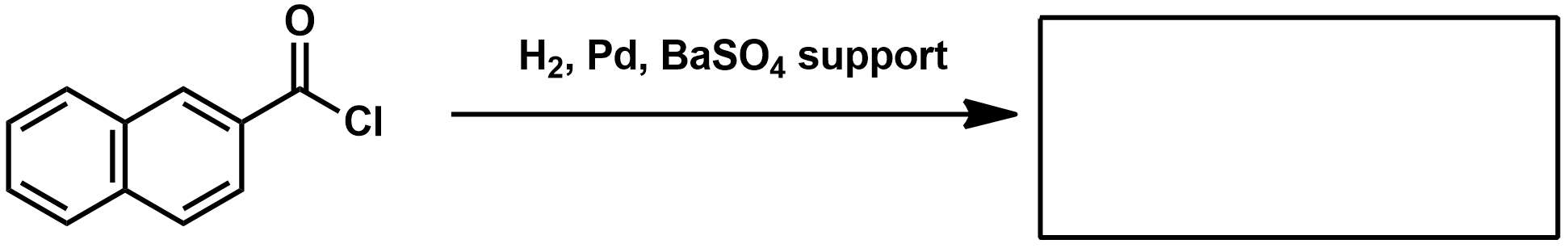
What is the Rosenmund Reaction?
Product of this reaction:
Aromatic Ring - O - R
+
H2 , Pd/C
Product of this reaction:
Aromatic Ring - CH2 - N-R2
+
H2 , Pd/C
Slide 26
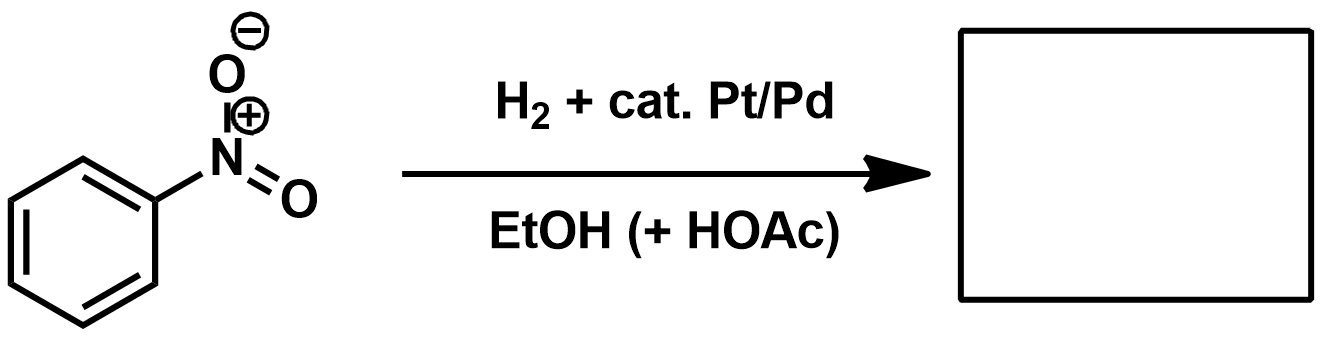
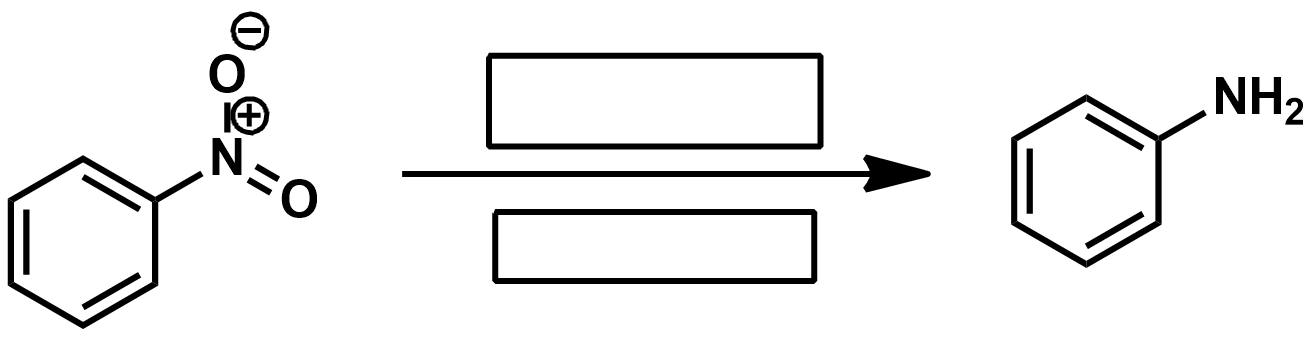
If you want to reduce a nitrile to an amine what should you use as a reagent?
If you want to reduce a nitrile to an aldeyde what should you use as a reagent?
Mechanism of thiocarbonyl formation?
What is the Barton-McCombie Radical deoxygenation Reaction?
What do you need to use to get an alcohol to an alkane?
Slide 32
What do you need to use to get halide to an alkane?
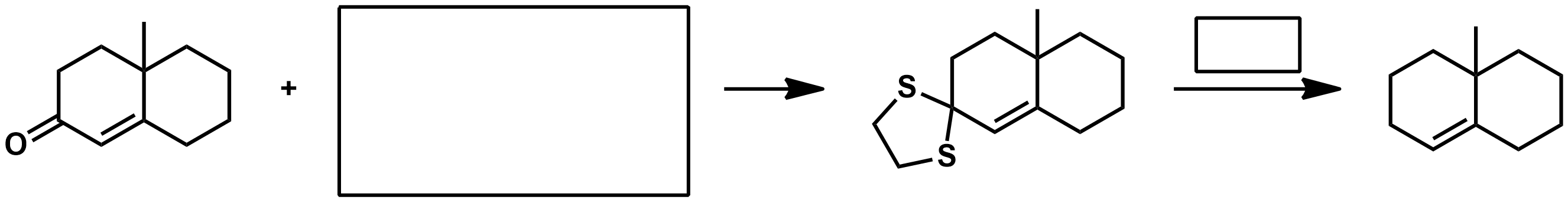
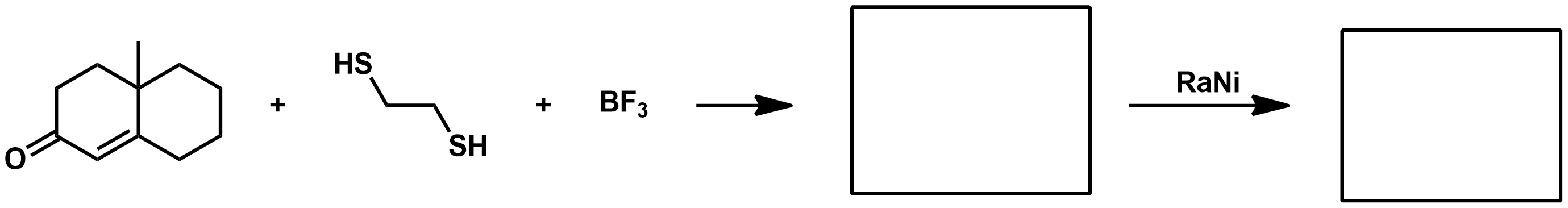
What does the Mozingo reaction do?
Missing KOH in reagent
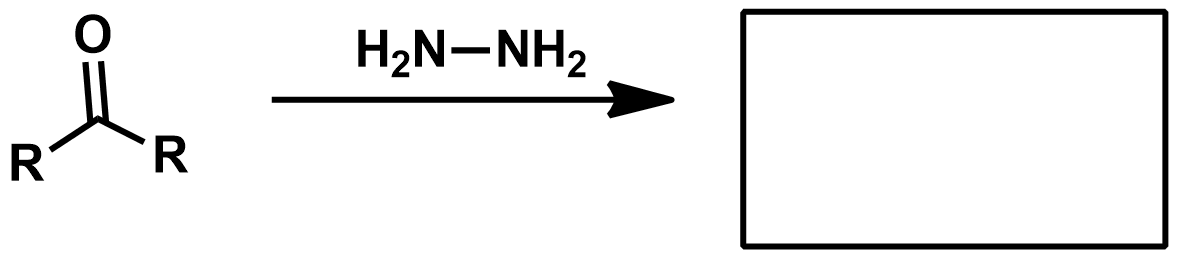
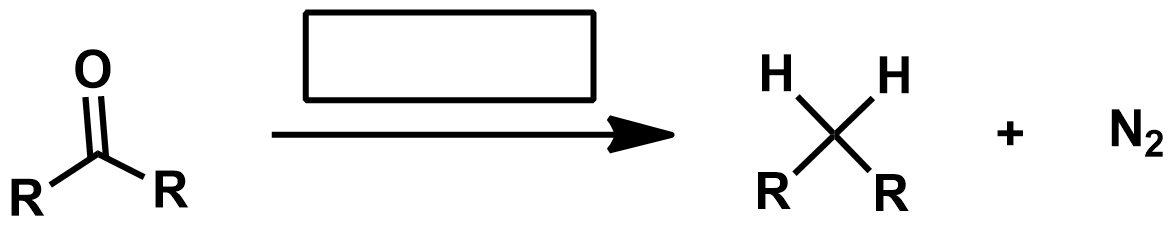
Missing KOH as reagent
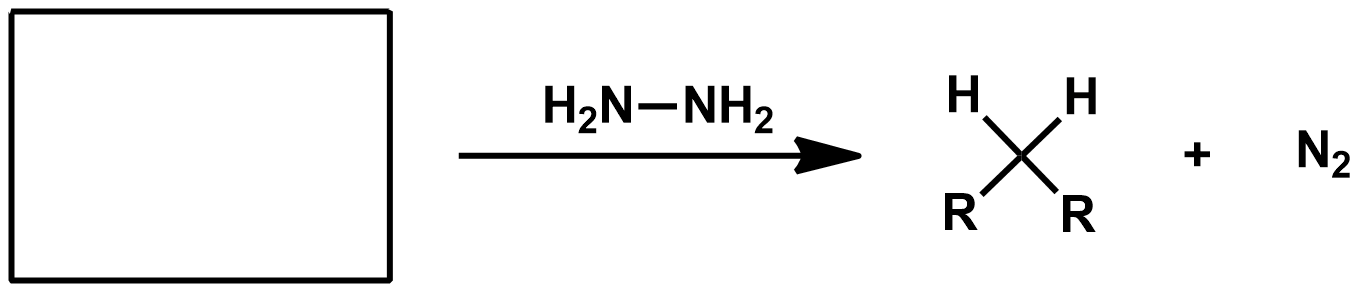
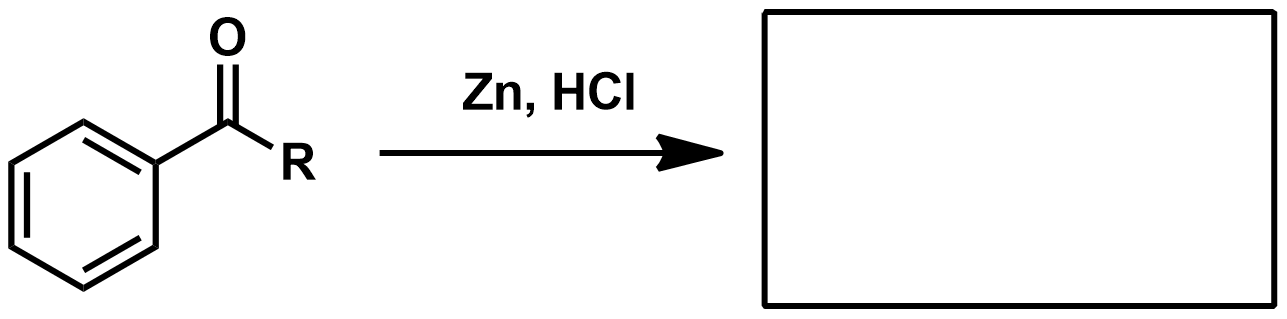
How can you go from carboxylic acid or acid chloride to alkane?
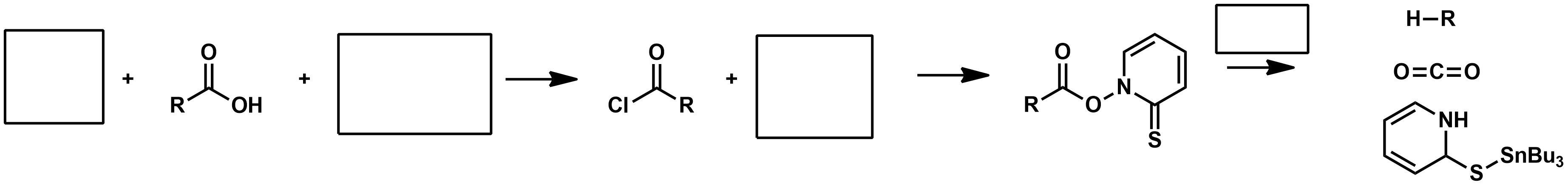
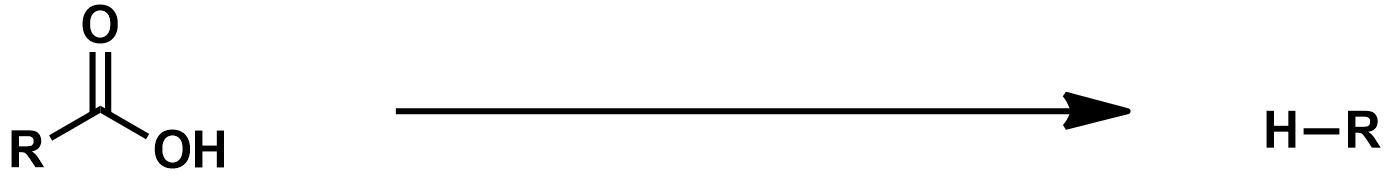
Mechanism Slide 35 + 36
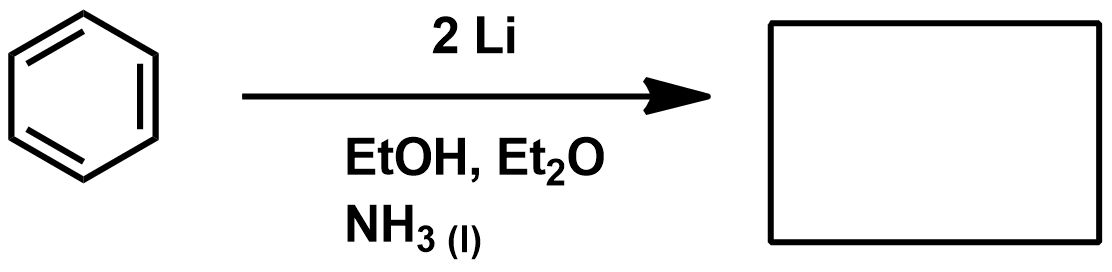
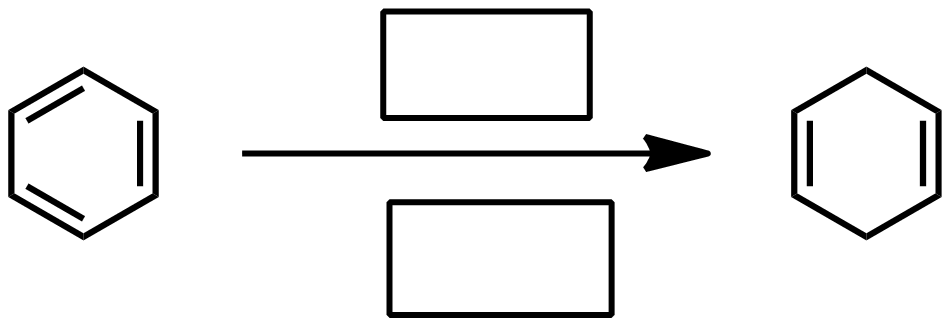
Birch reduction mechanism
EDG for birch reduction
EWG for birch reduction
Incompatible groups for birch reduction
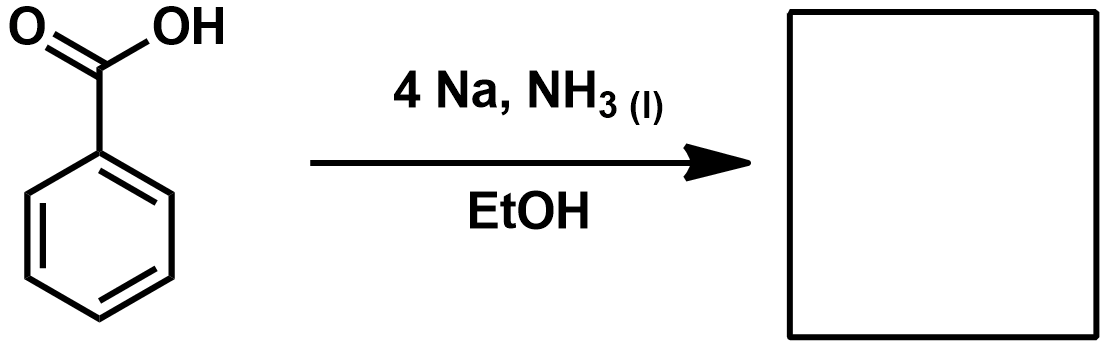
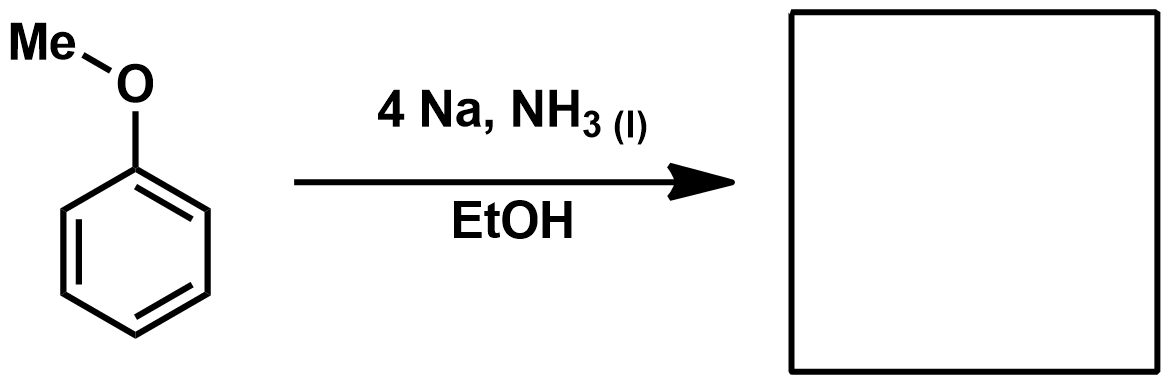
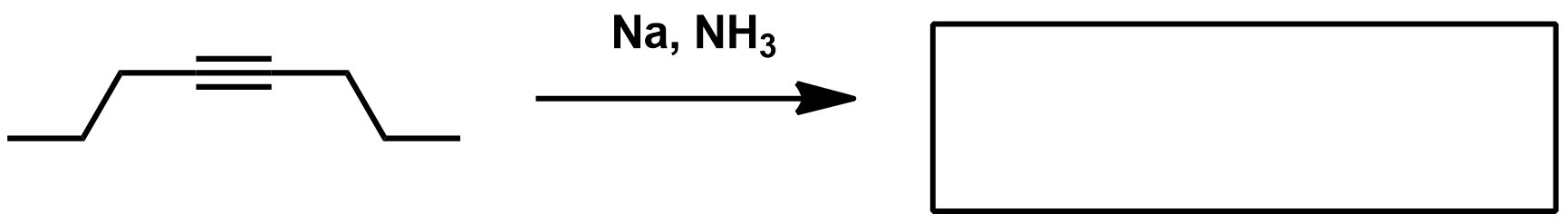
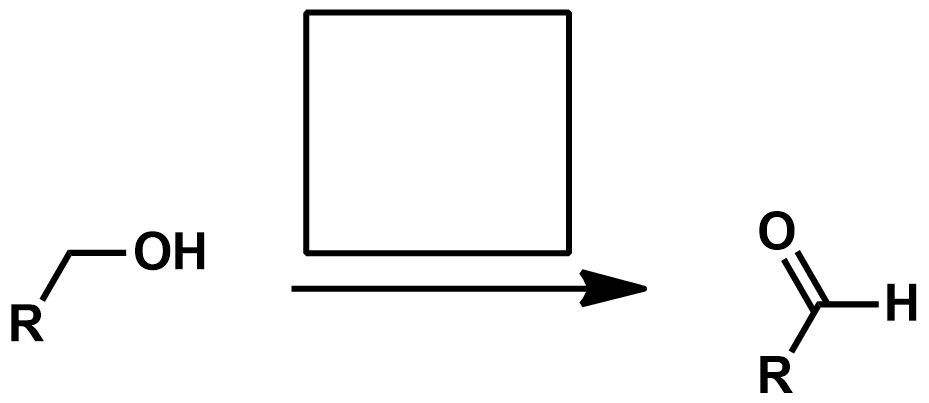
Reagents for Pinnick/Lindgren Oxidation
Swern Ox
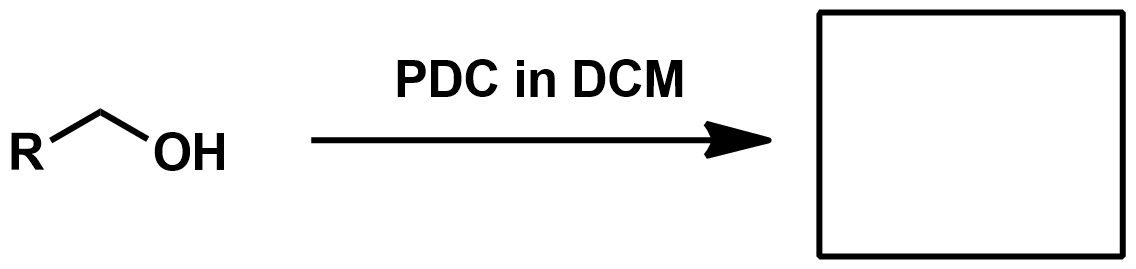
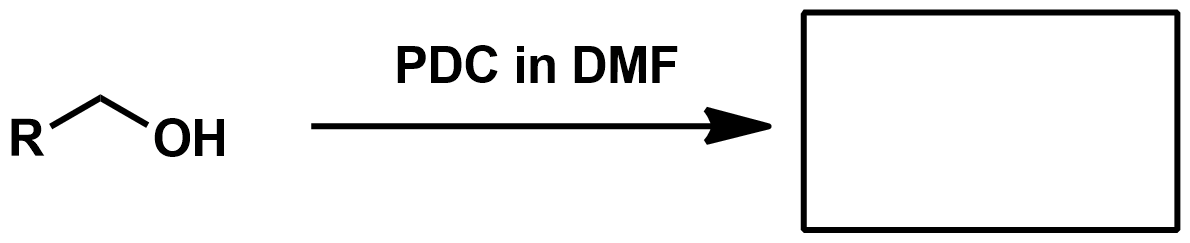
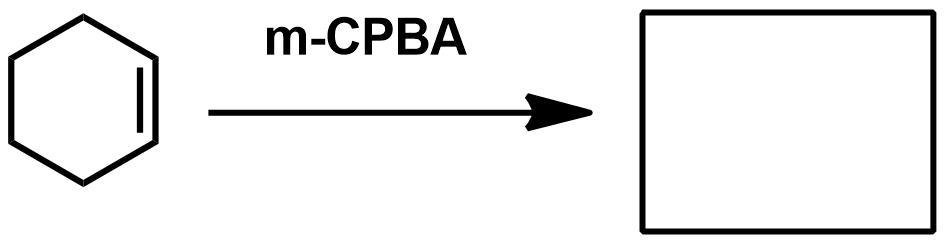
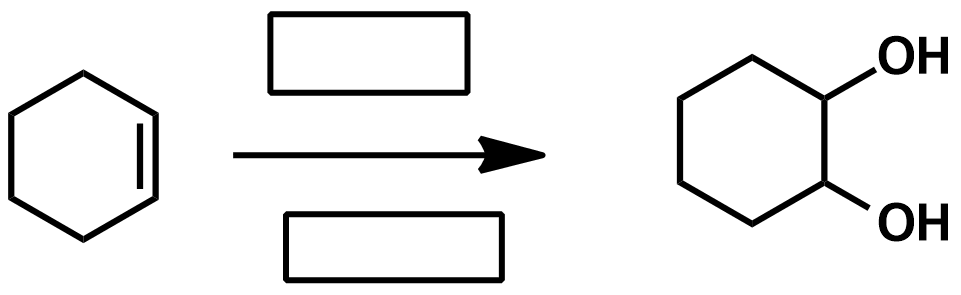
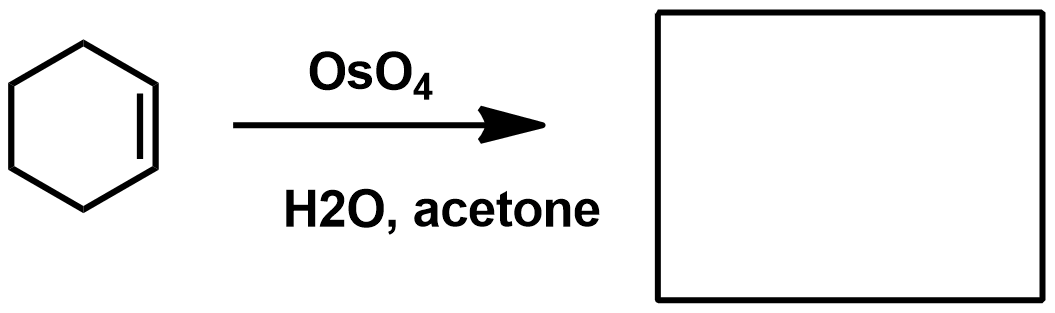
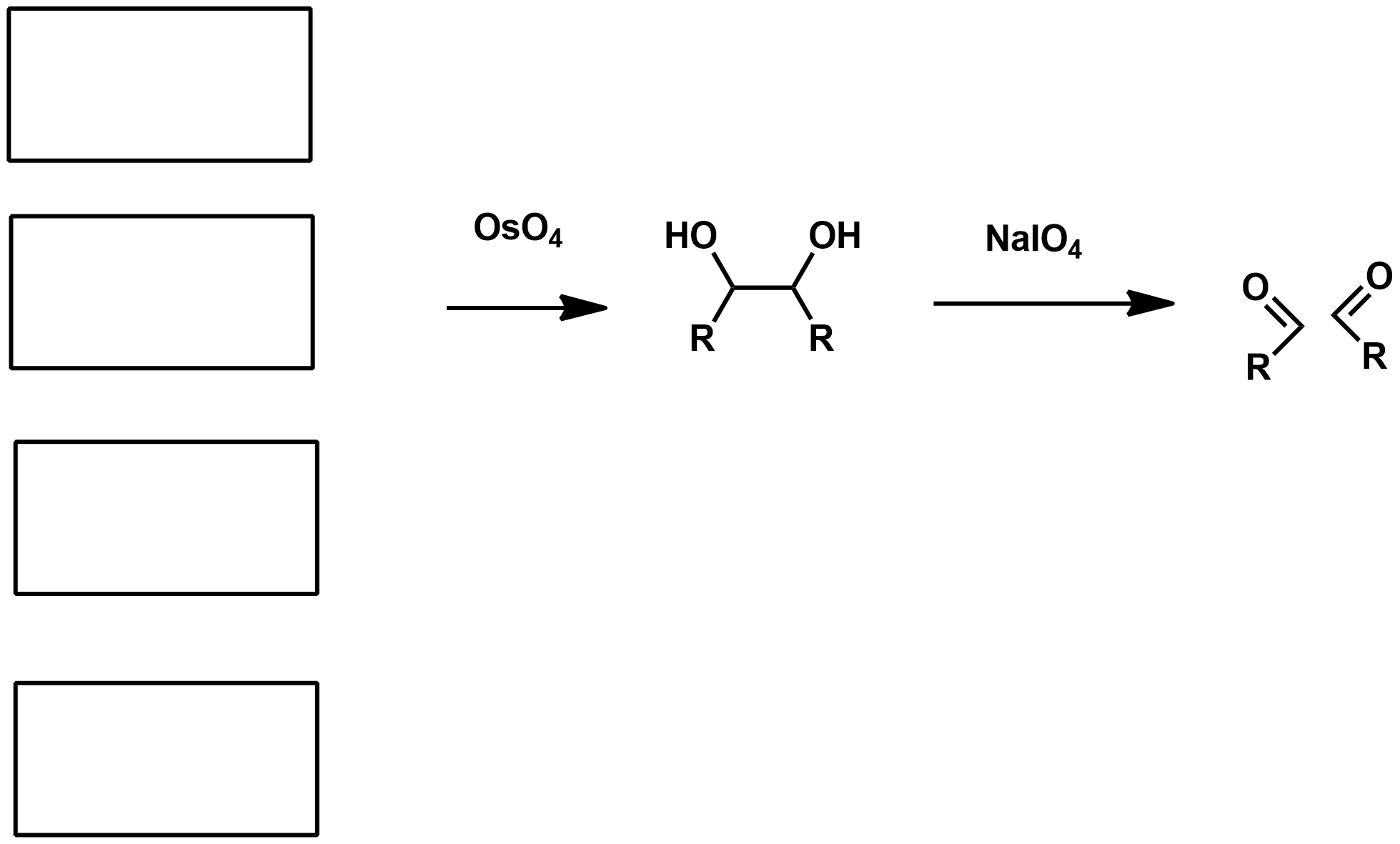
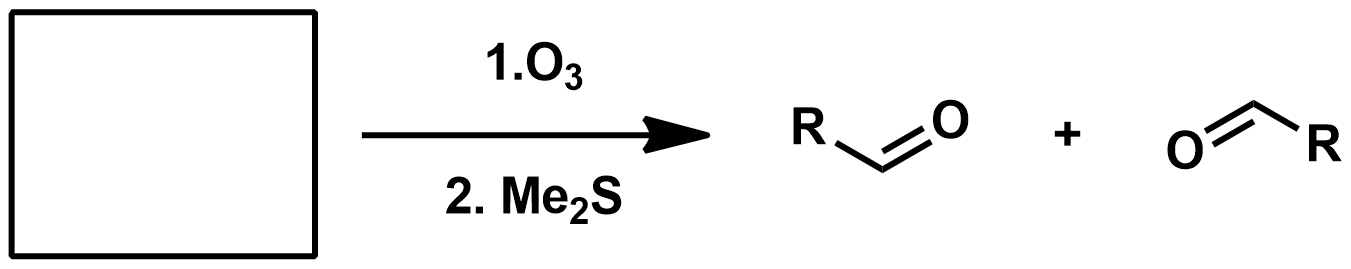
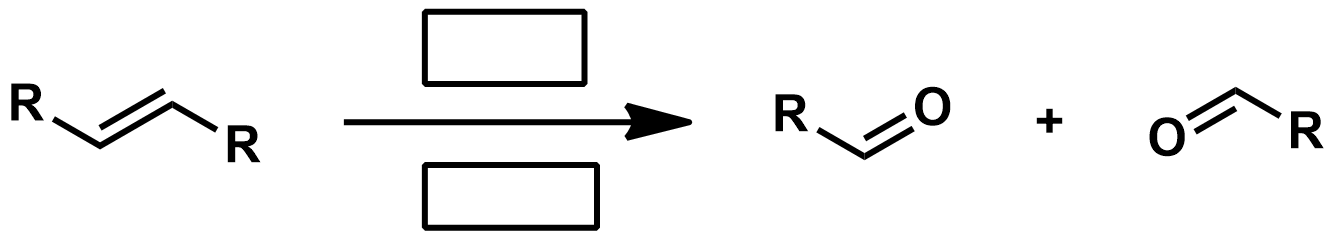

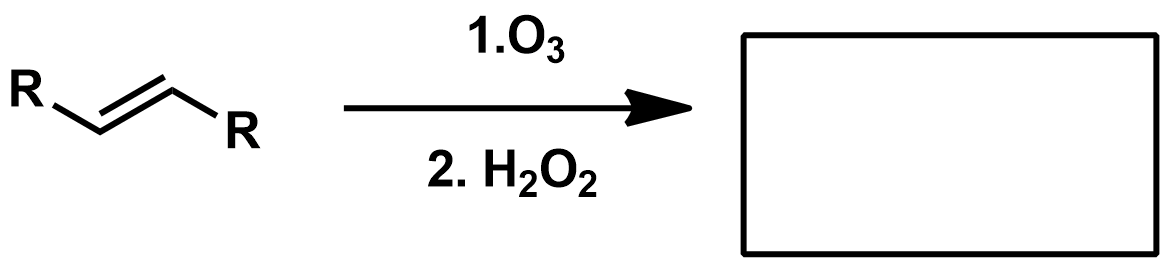
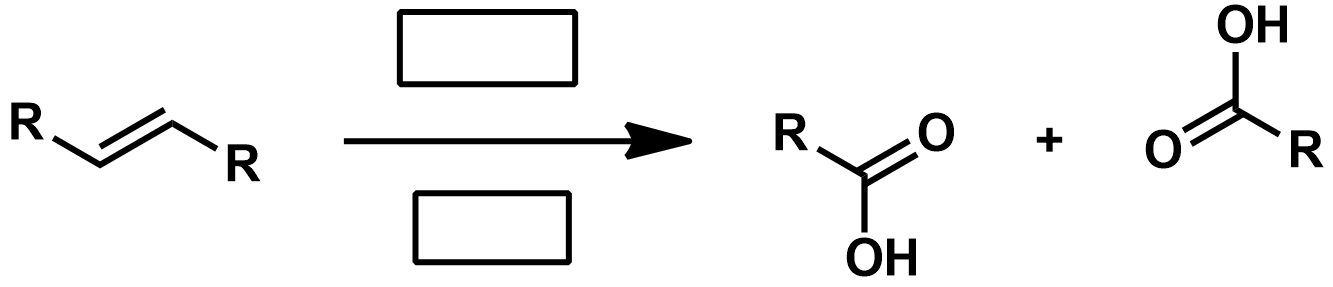
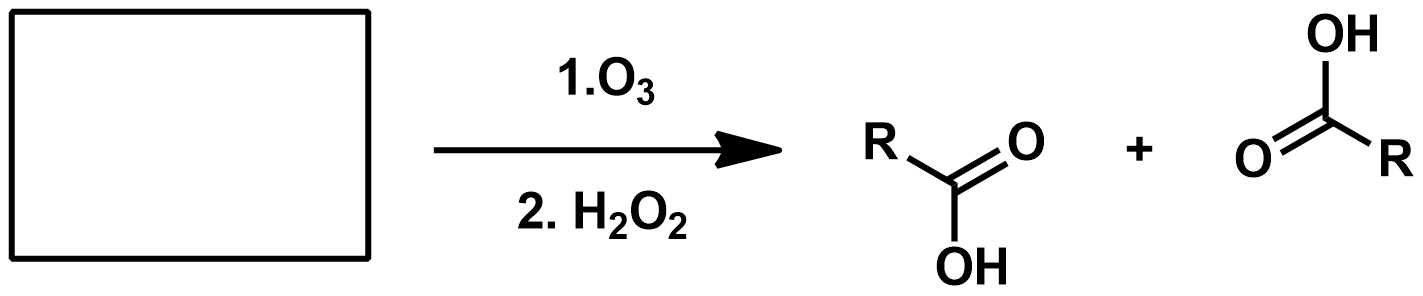
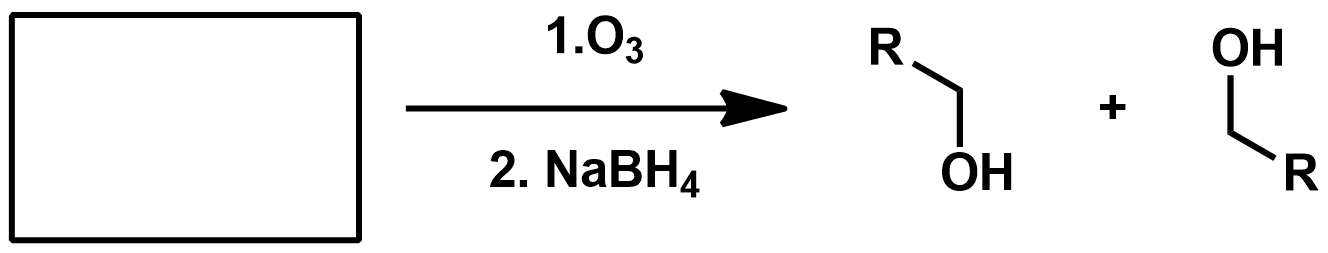
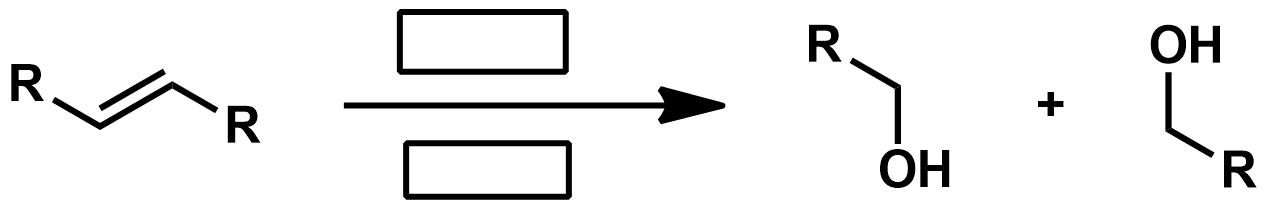

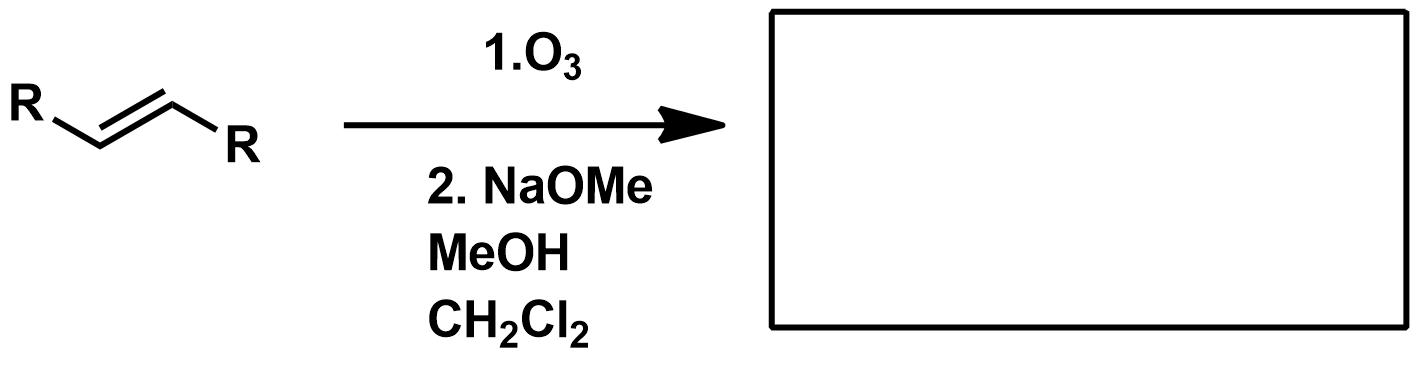
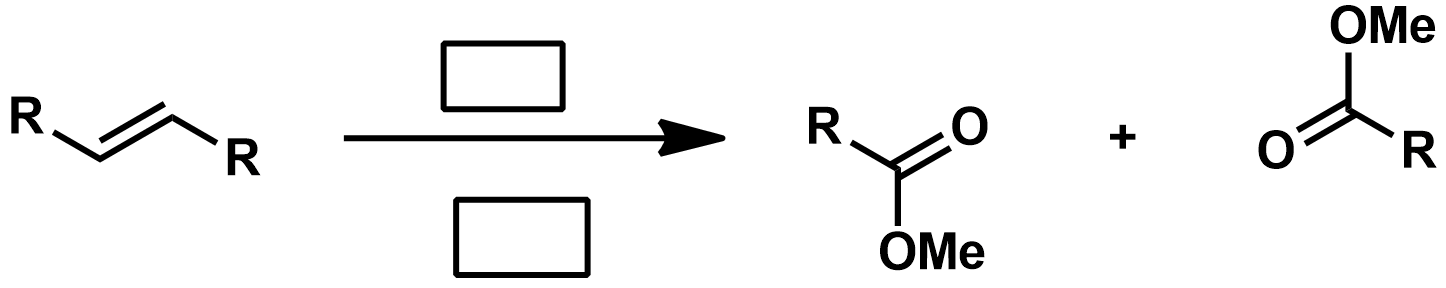
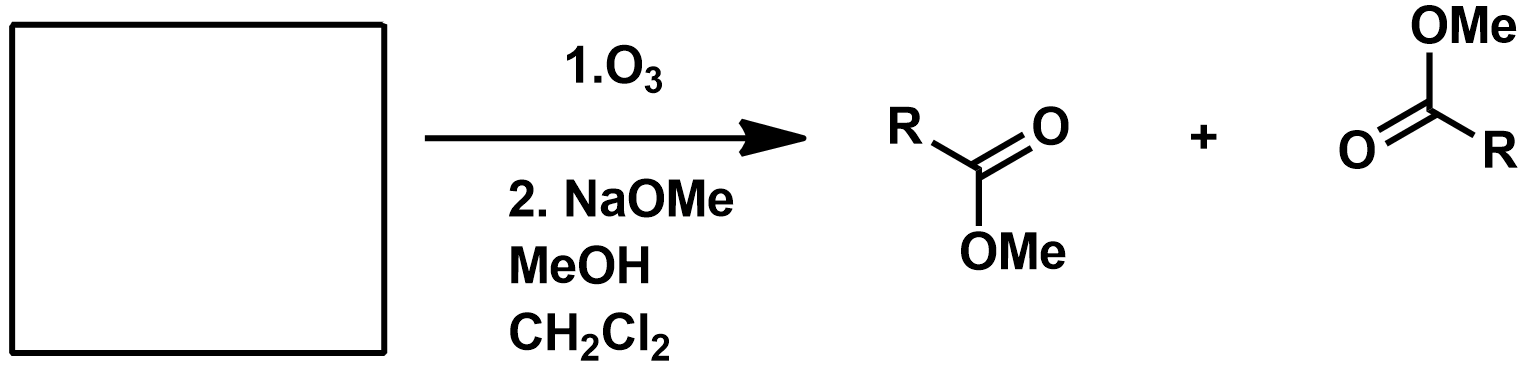
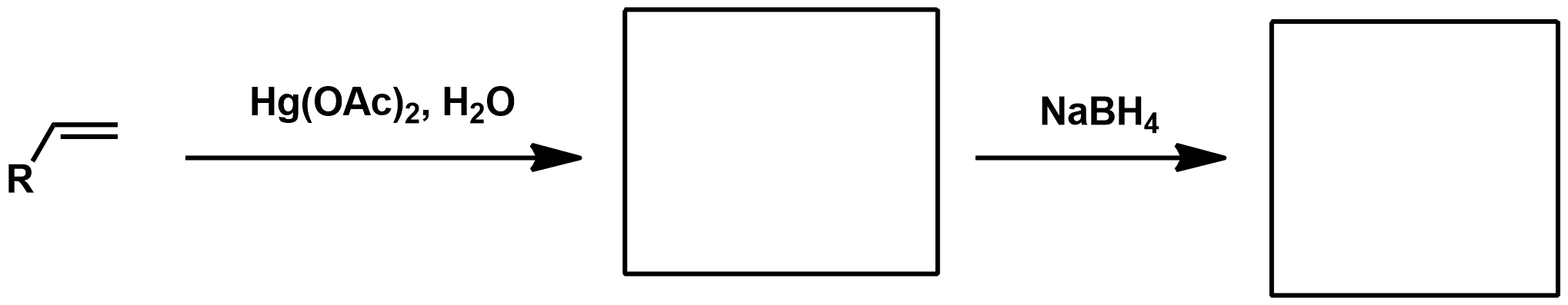
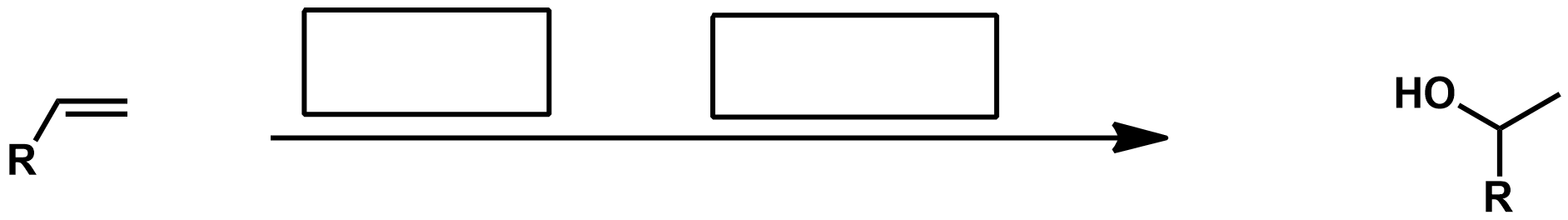
Slide 55
Hydroboration
Slide 56

 Ocultar acertos
Ocultar acertos