Fechar

What is the relative mass of the proton ?
What is the relative mass of the neutron ?
What is the relative mass of the electron ?
What is the mass number ?
What is the atomic number ?
What is an isotope ?
Give a definition for the relative atomic mass of an element
Give a definition for the relative formula mass of a compound
What is one mole of a substance ?
Elements and compounds can be detected and identified using instrumental methods. List the advantages of using instrumental methods.
What type of chemical analysis can be used to identify additives in foods.
Why do you use pencil and not pen to draw the base line in paper chromatography
Suggest why it is important to be able to identify the colour additives in food.
Describe how gas chromatography works.
What is the output of the gas chromatography column linked to ?
What do the number of peaks on the output of the gas chromatograph tells us ?
What is the retention time ?
What part of the mass spectrometer graph gives the molecular mass of a substance ?
Why do different compounds separate in a gas chromatography column?
In what part is the mixture separated ?
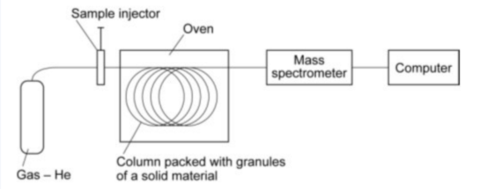
Why is helium used as a carrier gas in chromatography?
When a blood sample is taken from an athlete the sample is often split into two portions. Each portion is tested at a different laboratory.
Even though no atoms are gained or lost in a
chemical reaction, it is not always possible to obtain the calculated amount of a product , give 3 reasons why ?
aka : why is the actual yield always less than the theoretical yield ?
What is the amount of product obtained in a chemical reaction called ?
What formula is used to calculate the % yield ?
What is the symbol for a reversible reaction?
Give an example of a reversible reaction ?

 Ocultar acertos
Ocultar acertos