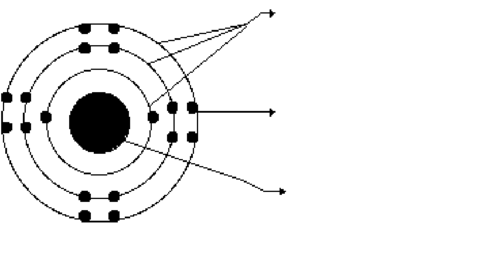
GCSE Chemistry FlashCards sobre Chemistry 1 Atoms, Elements and compounds Core GCSE, criado por Chloe Roberts em 21-04-2014.
Pin adicionado em
197
5
0
|
|
Criado por Chloe Roberts
mais de 10 anos atrás
|
|
Fechar